Eli Lilly and Company issues a voluntary nationwide recall of one lot of GLUCAGON® Emergency Kit due to Loss of Potency. Warning – A person with severe hypoglycemia who injects this faulty formulation will experience worsening hypoglycemia.
Eli Lilly and Company is voluntarily recalling lot D239382D, Expiration April 2022, of Glucagon Emergency Kit for Low Blood Sugar (Glucagon for Injection, 1 mg per vial; Diluent for Glucagon, 1 mL syringe).
Loss of Potency due to manufacturing process issue
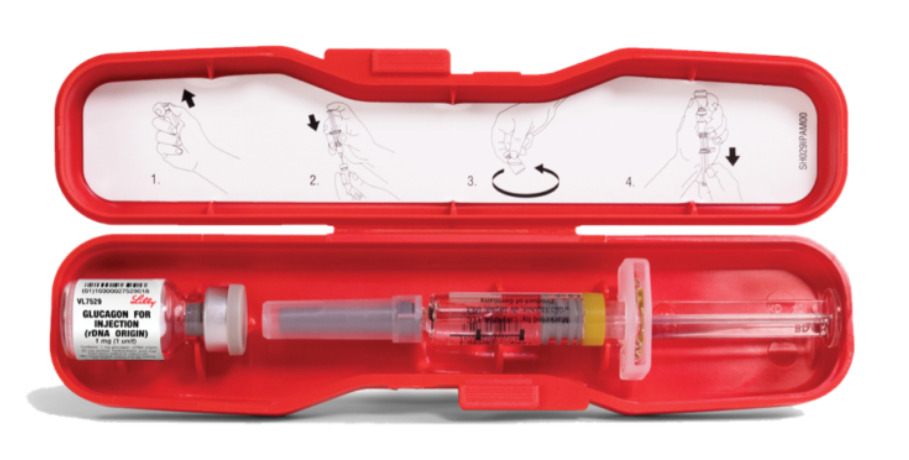
The Eli Lilly injectable glucagon kit (pictured here), usually contains a syringe with diluent and a vial with powdered glucagon. The user needs to put the diluent into the vial with the powdered glucagon and reconstitute it before injecting it.
However, a product complaint from a user found that the vial of Glucagon was in liquid form instead of powder form. Associated with this useful product complaint, the patient who was given this glucagon vial content, experienced worsening hypoglycemia and also reported subsequent seizures.
The use of the liquid form of this product may fail to treat severe low blood sugar due to loss of potency.
It is well known that severe hypoglycemia in people with diabetes, if not promptly treated and reversed, can potentially cause adverse health consequences ranging from transient, minor complaints to neurological damage, seizures, and even death. Eli Lilly’s investigation indicates that the liquid in this Glucagon vial could be related to a problem with the manufacturing process.
Please help get the word out
The Eli Lilly Glucagon product is packaged in a kit containing 1mg of freeze-dried (lyophilized) product in a 3 mL vial and a pre-filled diluent syringe. The affected Glucagon Emergency Kit lot is D239382D and the expiration date is April 2022 (label expiry date: 04 2022). The lot number can be found on the label of the kit as well as the vial (refer to the complete FDA warning below). The lot was distributed nationwide to wholesalers and retailers.
Contact Info for Consumers
Consumers in possession of Glucagon Emergency Kit lot D239382D should contact The Lilly Answers Center at 1-800-LILLYRX (1-800-545-5979) for return and replacement instructions for the product (hours of operation are Monday- Friday, 9AM – 7PM EST) and should contact their health care provider for guidance. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product.
Lilly is notifying its distributors and customers by written communication and is arranging for the return and replacement of all recalled products. Wholesalers and Distributors with an existing inventory of Glucagon Emergency Kit lot D239382D should cease distribution and quarantine the product immediately.
Read complete FDA Announcement Here
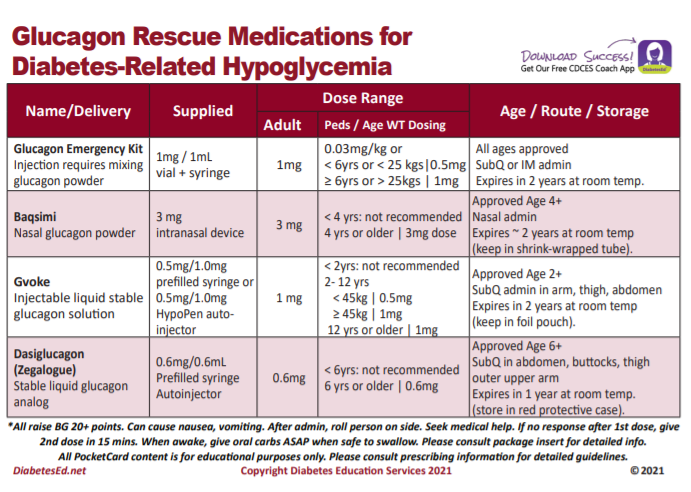
FREE Glucagon PocketCards
There are now 4 different glucagon formulations to choose from.
This free card details the different formulations available, from the injectables to the nasal powder formulation.
The backside includes teaching and hypoglycemia prevention strategies, along with the different official levels of hypoglycemia (for your certification study preparation).
Join us live on November 11th for the upcoming
Meritus Health’s Virtual Diabetes Conference
Diabetes in the 21st Century with Coach Beverly

Join us live on November 11th from 8:00 am to 4:00 pm for our Virtual Conference: Diabetes in the 21st Century | 6.5 CEs
This conference offers comprehensive presentations on care of a person with diabetes examining a variety of evidence-based topics to aid in the care of a person with diabetes.
Click here to download the program flyer.
Location: Virtual
Fees: No charge for Meritus Health Employees. $50.00 for Non-Meritus Health Participants Meritus Health Employees: Please register via Healthstream, using keyword search “21st Century” or by clicking here.
Cancellation Policy: If you must cancel, please notify Ruth Leizear by phone at 301-790-8619 or toll free at 888-803-1518.
Check-in: Check-in starts at 8:00 a.m., please log in 15 minutes prior to the first presentation
Accreditation:
- Registered Nurses: Nursing Education Department of Meritus Health is accredited with distinction as a provider of nursing continuing professional development by the American Nurses Credentialing Center’s Commission on Accreditation.
6.5 ANCC Contact Hours will be awarded; No partial credit will be given. - Dieticians: Approved for 6.5 CPE II for Registered Dietitians by the Commission on Dietetic Registration.
Click here to download the program flyer.
Instructor: Beverly Thomassian RN, MPH, CDCES, BC-ADM is a working educator and a nationally recognized diabetes expert.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Accreditation: Diabetes Education Services is an approved provider by the California Board of Registered Nursing, Provider 12640, and Commission on Dietetic Registration (CDR), Provider DI002. Since these programs are approved by the CDR it satisfies the CE requirements for the CDCES regardless of your profession.*
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.