Question of the Week & Rationale
Our “Question of the Week” is created weekly by Coach Beverly to cover a variety of Diabetes related topics. The questions are designed to keep you current and prepare you for the CDCES® Exam.
To sign up to receive the question of the week, download our free CDCES Coach App or join our Facebook Page.
This week’s Rationale: Question of the Week – January 23rd – test your knowledge before seeing answer below!
What a perfect way to information share and bring new concepts to light. We appreciate our community of diabetes educators and the opportunity to keep learning together!
Question:
A new biosimilar insulin, lispro (Admelog) is now FDA Approved. Which of the following statement is true about biosimilar insulins?
a. Biosimilar has the same meaning as generic
b. Biosimilar means that the product is highly similar
c. Biosimilar means the structure is slightly different, but the function is similar
d. Biosimilar means the function is slightly different, but the structure is similar
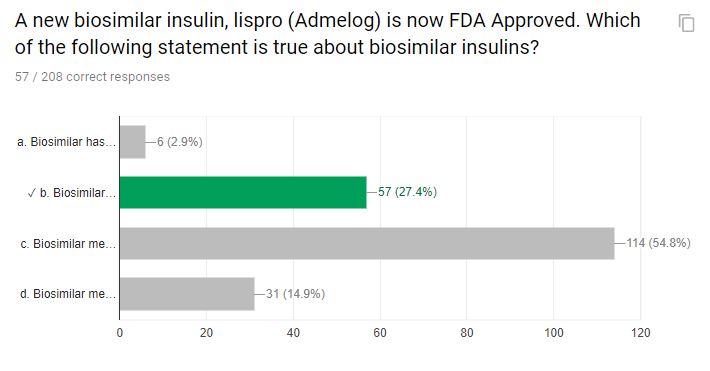
Correct Answer: b. Biosimilar means that the product is highly similar
Rationale: As the patents expire on our commonly used insulins, plan to see more biosimilar insulin products on the market. Since these biosimilar insulins are usually priced more competitively, expect to see insurance companies transferring patients to these less expensive insulins. Patients may feel uncomfortable with this change and providing them with information on bio similarity standards may help ease concerns. Below are excerpts from the FDA Website on the definition of biosimilar and highly similar.
What is a biosimilar product?
A biosimilar is a biological product that is highly similar to and has NO clinically meaningful differences from an existing FDA-approved reference product.
What does it mean to be “highly similar”?
Minor differences between the reference product and the proposed biosimilar product in clinically inactive components are acceptable. For example, these could include minor differences in the stabilizer or buffer compared to what is used in the reference product. Any differences between the proposed biosimilar product and the reference product are carefully evaluated by FDA to ensure the biosimilar meets FDA’s high approval standards.
A manufacturer developing a proposed biosimilar demonstrates that its product is highly similar to the reference product by extensively analyzing (i.e., characterizing) the structure and function of both the reference product and the proposed biosimilar. State-of-the-art technology is used to compare characteristics of the products, such as purity, chemical identity, and bioactivity. The manufacturer uses results from these comparative tests, along with other information, to demonstrate that the biosimilar is highly similar to the reference product.
As mentioned above, slight differences (i.e., acceptable within-product variations) are expected during the manufacturing process for biological products, regardless of whether the product is a biosimilar or a reference product. For both reference products and biosimilars, lot-to-lot differences (i.e., acceptable within-product differences) are carefully controlled and monitored.
Read more about this on the FDA Website
Download our updated FREE Injectables PocketCard
Or join our Medication Update webinar!
Meds Update 2018– Earn 1.5 CE – $29
Live webinar session February 19th, 2018 at 11:30 a.m. – 1:15 p.m. PST
Feeling overwhelmed by all the new recently approved diabetes medications? Two bio-similiar insulins are now available and another GLP-1 RA was just approved. Plus, 2 new combo oral meds are now available.
If you want cutting edge information on the latest pharmacology and hospital glucose management, we highly recommend this Meds Update.









