Recently, metformin was in the news regarding a potential risk of N-Nitrosodimethylamine (NDMA) contamination during medication production. The Food and Drug Administration (FDA) tested Metformin products to evaluate NDMA levels.

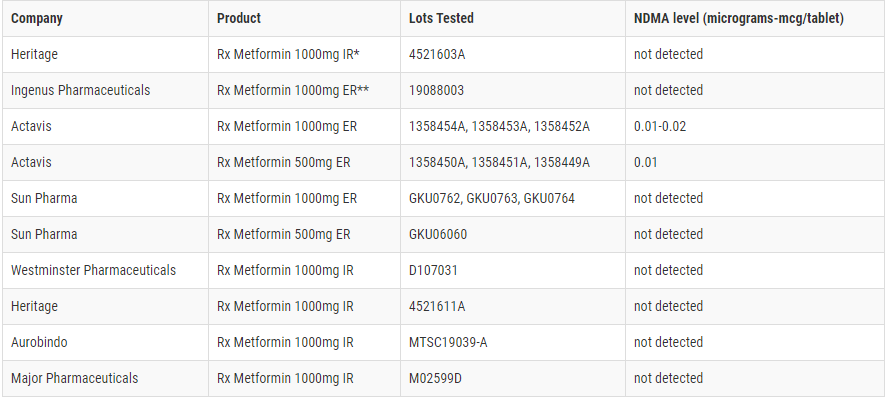
In Metformin’s products, NDMA is not listed under the active pharmaceutical ingredients. However, after some testing, the FDA did find “low levels” of NDMA in a few Metformin products (see chart below).
“Most metformin drug products tested showed no detectable levels of NDMA, while others showed low levels of NDMA,” FDA said, noting that the low levels are similar to what one would be exposed to from grilled or smoked meats.
The FDA advises that “patients should continue taking Metformin to keep their diabetes under control. ”
Of the products tested, both Actavis’ Rx Metformin 1000mg ER and Rx Metformin 500mg ER contained trace amounts of NDMA. The FDA explained that these low levels found (at 0.01-0.02 micrograms-mcg/tablet) are akin to exposure to NDMA through grilled or smoked meats. The daily intake limit for NDMA is 0.096 micrograms, which the FDA regards as “reasonably safe for human ingestion based on lifetime exposure.”
So far, the FDA has not recommended Metformin recalls in the US. Though other countries, like Singapore, have recalled a few Metformin products containing NDMA “above the internationally acceptable level.”
Click here to read more. Download Diabetes Medication PocketCards here.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]









