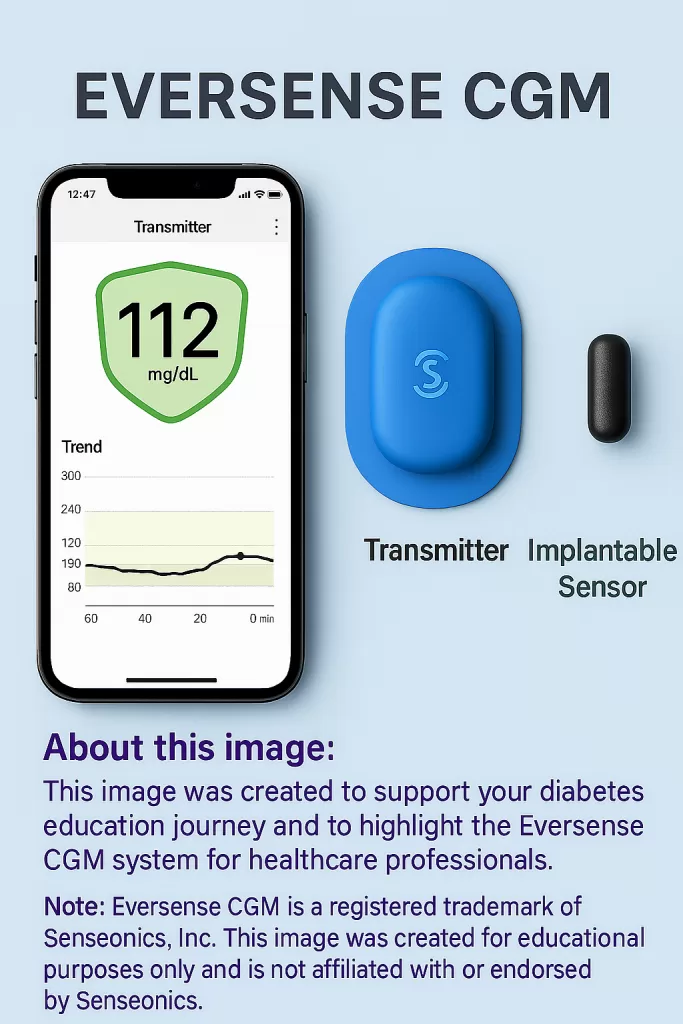
Do your clients accidentally knock off their continuous glucose monitor (CGM) or complain about how frequently they need to be replaced? If so, did you know there is an implanted CGM option available?
According to the ADA 2025 Standards of Care, CGMs are now the standard of care for individuals using insulin therapy or experiencing hypoglycemia.¹ There are many similarities between the available CGMs, but the Eversense CGM by Senseonics stands out due to some striking differences. As the diabetes education specialist, you can highlight the similarities and differences between CGMs so people can make a personalized and informed decision about which device works best for them.
Get to Know Eversense 365
The Eversense 365 is an implanted CGM (iCGM) used to monitor glucose in individuals 18 or older with diabetes. It is currently the only FDA-approved implantable glucose sensor in the United States. The Eversense system has launched the current 365-day sensor, which was approved for use in October 2024.
This system consists of a small sensor implanted into the subcutaneous tissue of the outer upper arm. A smart transmitter is placed over the inserted sensor to transmit data to a compatible smartphone. Glucose accuracy is similar to other prescription CGMs on the market.²
This system requires initial calibrations when starting a new transmitter. Calibrations must be completed within 36 hours.³
Initial calibration schedule:
- #1 – After the 24-hour warm-up phase
- #2 – 2-12 hours after the first calibration (Glucose readings will then begin to display on the app)
- #3 – 2-12 hours after the second calibration
- #4 – 2-12 hours after the third calibration
Ongoing calibration schedule:
- 1 calibration every 24 hours for 13 days
- 1 weekly calibration for the life of the sensor
Key Components of Eversense 365
The three key components of the Eversense 365 iCGM system are an implantable sensor, a smart transmitter, and a phone app that displays and shares data.
Implantable Sensor
- Sensor has a 365-day lifespan.
- Subcutaneous placement by trained provider.
- Sensor precautions:
- Do not inject insulin or insert an insulin pump infusion site within 4 inches of the iCGM sensor.
- Do not use lithotripsy, diathermy, electrocautery, or receive vaccinations near the inserted sensor, as this may cause damage to the sensor.
- The sensor can be worn in an MRI under specific conditions (see Eversense 365 Glucose Monitoring System User Guide for more information).
Smart Transmitter
- The transmitter is removable and rechargeable.
- Glucose data is transmitted every 5 minutes to the Eversense 365 app after the second calibration.
- Creates vibration alerts for high and low glucose readings.
- The transmitter must be removed before MRI, CT, or x-ray.
Mobile App & Data Sharing:
- Eversense 365 app displays real-time glucose trends.
- The app allows individuals to share glucose data with family, friends, and healthcare providers.
Eversense 365 Eligibility, Benefits, & Risks
The Everesense iCGM is approved for adults aged 18 and older with diabetes. This is available by prescription only. Medicare coverage for CGM requires a diagnosis of diabetes and being treated with insulin, or having two or more level 2 hypoglycemic episodes (<54 mg/dL).⁴ Coverage with commercial insurance varies, and Medicaid coverage varies from state to state.
Eversense 365 Benefits
- Decreased adhesive allergies
- The silicone-based adhesive on the transmitter can result in fewer skin reactions.
- Less frequent sensor changes
- The sensor is placed only once a year.
- Decreased wasted sensors falling off or failing early
- The transmitter is removable and can be replaced easily if knocked off.
- Decreased rate of compression lows
- Fewer compression lows have been noted⁵
- No worry about vitamin C or acetaminophen interfering with CGM readings
- These do not interfere with iCGM glucose readings like with other CGMs.
Contraindications & Precautions
- Do not use in individuals with known allergy to:
-dexamethasone or dexamethasone acetate
-any component of the sensor, transmitter, or adhesive - Mannitol or sorbitol (intravenous, irrigation, or peritoneal dialysis solution, but not sorbitol in the diet) can cause falsely elevated glucose levels.
- The tetracycline antibiotic class can cause falsely low glucose readings
- Always check fingerstick blood glucose for very high or very low glucose readings before making a treatment decision
- The system has not been tested on:
-Those who are pregnant
-Those under 18 years old
-Those taking certain medications: chemotherapy, anticoagulant therapy, or immunosuppressive therapy
-Those with active implantable devices (such as a cardiac defibrillator)³
Role of the Diabetes Education Specialists
Diabetes care and education specialists (DCES) are an essential source of information for those living with diabetes to help achieve glycemic goals with a personalized approach to technology. Individualized education and training on the Eversense 365 iCGM ensure the proper use of the system, including calibration and interpretation of the glucose data. Understanding the details of the Eversense 365 allows the DCES to efficiently help troubleshoot problems, leading to long-term adherence and maximizing the benefits of an iCGM to improve diabetes outcomes.
Ready for a closer look at how technology integrates into successful diabetes outcomes? Check out the Diabetes Ed Training Conferences for technology updates and more!
References
- American Diabetes Association Professional Practice Committee. (2025). Diabetes technology: Standards of care in diabetes – 2025. Diabetes Care, 48 (supplement 1), S146-S166. https://doi.org/10.2337/dc25-S007
- Danatech. (n.d.). Find & compare continuous glucose monitors. https://www.adces.org/education/danatech/glucose-monitoring/continuous-glucose-monitors-(cgm)/view-compare-cgms
- Senseonics. (2025) Eversense 365 Continuous Glucose Monitoring System User Guide.https://www.eversensecgm.com/user-guides/
- American Diabetes Association. (n.d.). FAQs on CGM coverage criteria changes in Medicare. https://diabetes.org/advocacy/cgm-continuous-glucose-monitors/faqs-medicare-coverage#:~:text=Medicare%20now%20allows%20people%20to,day%20to%20access%20a%20CGM.
- Christiansen, M. P., Klaff, L. J., Brazg, R., Chang, A. R., Levy, C. J., Lam, D., Denham, D. S., Atiee, G., Bode, B. W., Walters, S. J., Kelley, L., & Bailey, T. S. (2018). A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technology & Therapeutics, 20(3), 197. https://pmc.ncbi.nlm.nih.gov/articles/PMC5867508/
ReVive 5 Diabetes Training Program: A Person-Centered Approach to Diabetes Distress & Glucose Management
Join us live on July 15th and July 22nd, 2025 at 9:00 am PST
6+ CEs with Expanded Accreditation!

Join experts Larry Fisher, Ph.D., ABPP, Susan Guzman, Ph.D., and Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM, for this transformative two-part training on the ReVive 5 framework—an evidence-based approach that integrates emotional well-being and glucose data into person-centered diabetes care.
Grounded in the results of the EMBARK Trial, this program emphasizes the urgent need to assess and address diabetes distress, now recognized in the ADA Standards of Care as a critical component of care. You’ll gain practical tools and strategies used in the trial to support emotional well-being, meet clinical standards, and empower individuals on their diabetes journey.
Program Objectives:
Upon completion of this activity, participants will be able to:
- Identify the key differences between diabetes distress and depression and explain their impact on self-care behaviors.
- Apply evidence-based communication strategies to respond to diabetes distress screening results.
- Demonstrate how to analyze glucose patterns using meter and sensor data.
- Utilize the ReVive 5-step approach to integrate emotional and glucose management into diabetes care.
Team of Experts:
ReVive 5 is taught by a team of 3 Interdisciplinary Experts:



Lawrence Fisher, Ph.D., ABPP
Professor Emeritus, UCSF
Susan Guzman, PhD
Beverly Thomassian, RN, MPH, CDCES, BC-ADM
CEO of DiabetesEd Services
Faculty Bios & Disclosures
Program Faculty Disclosures:
Partners for Advancing Clinical Education (Partners) requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
Faculy Bios & Disclosures:
 Lawrence Fisher, Ph.D., ABPP, Professor Emeritus, UCSF
Lawrence Fisher, Ph.D., ABPP, Professor Emeritus, UCSF
- Consultant, advisor, and speaker for Eli Lilly
Speaker Interview:
Bio:
Dr. Fisher has been a professor in the Department of Family & Community Medicine at the University of California, San Francisco for over 25 years, and he is the Director of The Behavioral Diabetes Research Group at UCSF.
He has a Diplomate in Clinical Psychology from the American Board of Professional Psychology and is a former Associate Editor of Diabetes Care. He has conducted multiple cross-sectional and longitudinal NIH- and ADA-supported clinical research with adults with diabetes and their families.
His recent work focuses on diabetes distress and depression, disease management, and how adults and families struggle over time to manage chronic health conditions. He has won two major UCSF School of Medicine teaching awards, was nominated for the UCSF Postdoctoral Scholars Association Outstanding Mentorship Award, and has received a certificate from the American Psychological Association in “Recognition for Substantial Contributions to the Field of Family Psychology and Health.”
In 2012 he received the Richard Rubin Award from the American Diabetes Association. He maintains an active clinical practice at UCSF, has published over 190 peer-reviewed articles on diabetes and related topics, and frequently speaks to both professional and lay groups at local, national, and international meetings and workshops.
 Susan Guzman, PhD
Susan Guzman, PhD
- Consultant, advisor, and speaker for Abbot Labratories and Embecta
Dr. Guzman is a clinical psychologist specializing in diabetes. In 2003, Dr. Guzman co-founded the Behavioral Diabetes Institute (BDI), the first non-profit organization devoted to the emotional and behavioral aspects of living with diabetes.
At BDI, she serves as the Director of Clinical Education, developing and leading programs for people with diabetes and healthcare professionals. She has helped develop and facilitate diabetes distress group interventions for two NIH-funded research studies for adults with type 1 diabetes.
Dr. Guzman is passionate about helping to change the conversations in diabetes away from shame, blame, and judgment to those based on facts, empathy, and engagement. She has been part of a joint ADA/ADCES effort to address problematic language and messages in diabetes.
 Coach Beverly Thomassian RN, MPH, CDCES, BC-ADM
Coach Beverly Thomassian RN, MPH, CDCES, BC-ADMBeverly Thomassian has no financial disclosures
Speaker Interview:
Bio:
Expanded Accreditation
Activity Start and End Date: 7/15/2025 to 7/22/2027
Estimated time to complete the activity: 6 hours and 15 minutes
Jointly provided by Partners for Advancing Clinical Education and Diabetes Education Services
![]()
![]()
Joint Accreditation Statement:
 In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Diabetes Education Services. Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Diabetes Education Services. Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education:
Partners designates this enduring material for a maximum of 6.25 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Nursing Continuing Professional Development:
The maximum number of hours awarded for this Nursing Continuing Professional Development activity is 6.25 contact hours.
Pharmacy Continuing Education:
Partners designates this continuing education activity for 6.25 contact hour(s) (.625] CEUs) of the Accreditation Council for Pharmacy Education.
(Universal Activity Number – UAN JA4008073-9999-25-198-H01-P)
Type of Activity: Application
For Pharmacists: Upon successfully completing the activity evaluation form, transcript information will be sent to the NABP CPE Monitor Service within 4 weeks.
Dietitian Continuing Education:
This program offers 6.25 CPEUs for dietitians.
Interprofessional Continuing Education:
![]() This activity was planned by and for the healthcare team, and learners will receive 6.25 Interprofessional Continuing Education (IPCE) credit for learning and change.
This activity was planned by and for the healthcare team, and learners will receive 6.25 Interprofessional Continuing Education (IPCE) credit for learning and change.
Disclosure of Unlabeled Use:
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications. The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer:
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Instructions for Credit
Participation in this self-study activity should be completed in approximately 6 hours and 15 minutes. To successfully complete this activity and receive CE credit, learners must follow these steps during the period from 7/15/2025 to 7/22/2027.
- Review the objectives and disclosures
- Study the educational content in Online University
- After review of content, a module within the course in the Online University will list a link to Partners for Advancing Clinical Education’s website
- Visit Partners for Advancing Clinical Education website listed in course in the Online University
- Complete the activity evaluation through Partners for Advancing Clinical Education website
For additional information about the accreditation of this activity, please visit https://partnersed.com.
The use of DES products does not guarantee the successful passage of the certification exam. CBDCE and ADCES do not endorse any preparatory or review materials for the CDCES or BC-ADM exams, except for those published by CBDCE & ADCES.








