Rates of type 2 diabetes in youth continue to climb. Based on information from the SEARCH for Diabetes in Youth study, the incidence of type 2 diabetes in children increased by 4.8% per year from 2002 to 2015 and is expected to continue increasing. As of 2017, there were approximately 28,000 cases of type 2 diabetes in children in the U.S. By 2060, if current trends continue, that number is predicted to be approximately 220,000, with the majority of cases occurring in minority racial and ethnic groups such as Non-Hispanic Black people and Hispanic people.

Pediatrics have limited medication options:
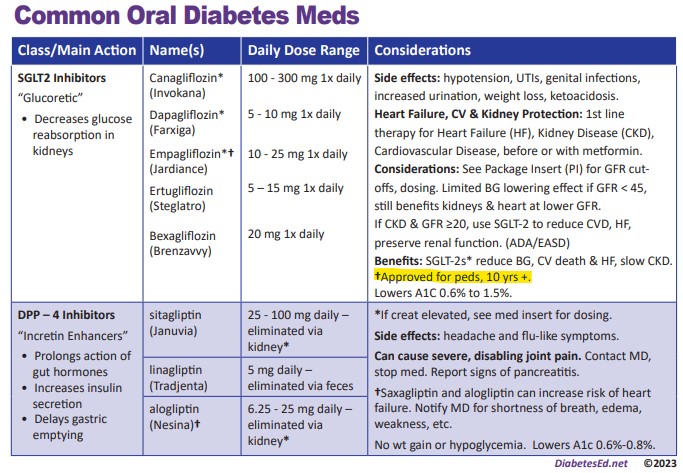
Compared to adults, children with type 2 diabetes have limited medication treatment options even though type 2 diabetes generally progresses more rapidly in youth. Metformin was the only oral medication approved for children 10 years and older until last month.
In late June, the U.S. Food and Drug Administration approved Jardiance (empagliflozin) and Synjardy (empagliflozin and metformin hydrochloride) as additions to diet and exercise to improve blood sugar management in children 10 years and older with type 2 diabetes. These approvals provide a new class of medicines to treat pediatric diabetes, in addition to the handful of other FDA-approved medications including; metformin, liraglutide, semaglutide, exentatide XR and insulin therapy.
Trials show significant glucose improvement
The safety and efficacy of empagliflozin in children were studied in a double-blind, randomized, placebo-controlled trial in 157 patients aged 10 to 17 years with inadequately controlled type 2 diabetes.
Results from the DINAMO phase III trial, in which Jardiance demonstrated a statistically significant reduction in the primary endpoint of change from baseline in A1c at 26 weeks compared with placebo in participants aged 10-17 years with type 2 diabetes. When added to other baseline treatments (diet, exercise, metformin, and/or insulin), Jardiance 10 mg and 25 mg pooled doses reduced A1c by 0.84% compared with placebo at week 26.
Common side effects in children treated with empagliflozin were generally similar to those reported in adults, except there was a higher risk of hypoglycemia among pediatric patients 10 years and older taking empagliflozin compared to placebo, regardless of whether they were taking other therapies for diabetes.
Not recommended for people with Type 1 Diabetes
Jardiance and Synjardy are not recommended in patients with type 1 diabetes because of an increased risk of diabetic ketoacidosis. Jardiance and Synjardy are also not recommended for use to improve blood sugar control in patients with severe kidney problems and should not be used in patients who previously have had a serious allergic reaction to them. Synjardy must not be used in patients with metabolic acidosis or diabetic ketoacidosis (increased ketones in the blood or urine).
Jardiance and Synjardy received priority review designations. A priority review designation directs overall attention and resources to the evaluation of applications for drugs that, if approved, would be significant improvements in the safety or effectiveness of the treatment, diagnosis or prevention of serious conditions.
Medication PocketCards Updated to Reflect this change
We have updated our ePocketCards with the new approval and plan to go to press this month to update our printed version too. We will keep you posted!
Want to learn more about this practice question?
Join us LIVE in San Diego for our DiabetesEd Training Conference
October 11-13th, 2023

Two Registration Options
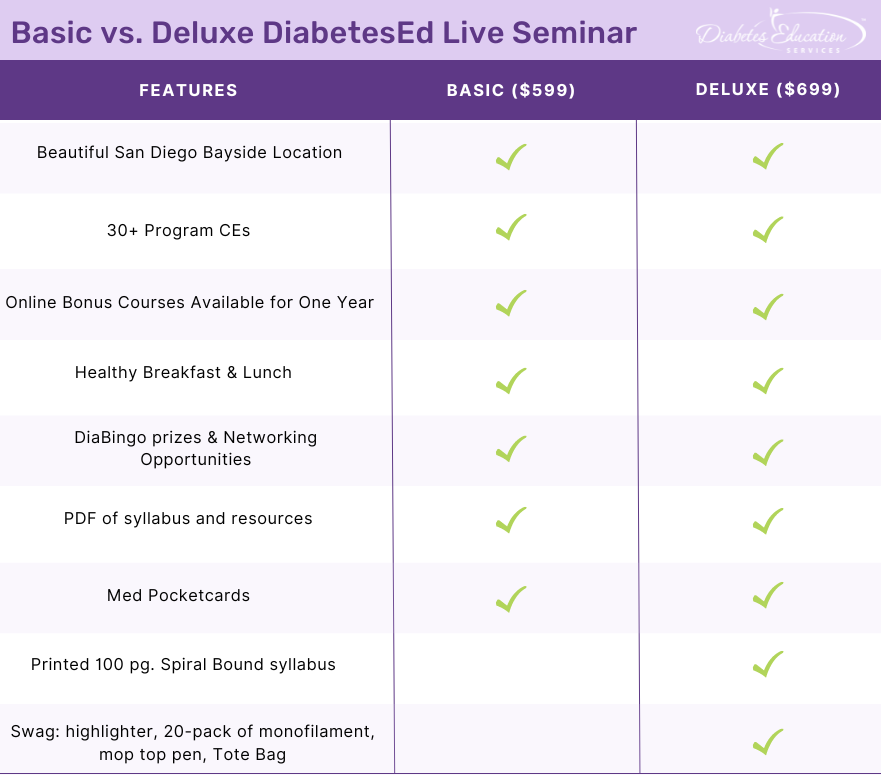
Join Coach Beverly and Team for two and a half days of knowledge-sharing, fun, networking, games with prizes, and “aha” moments in beautiful San Diego.
You don’t want to miss this one-of-a-kind learning opportunity. Get away from all those daily responsibilities and immerse yourself in a fun and intensive conference with plenty of networking opportunities.
Attendees will leave this conference with new tools and a more complete understanding of the latest advances in diabetes care, from medications to technology to Medical Nutrition Therapy!
Bring your colleagues and enjoy our friend discount.
Our team expertly translates the complex science of diabetes into understandable terms while keeping it real, practical, and fun.
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.