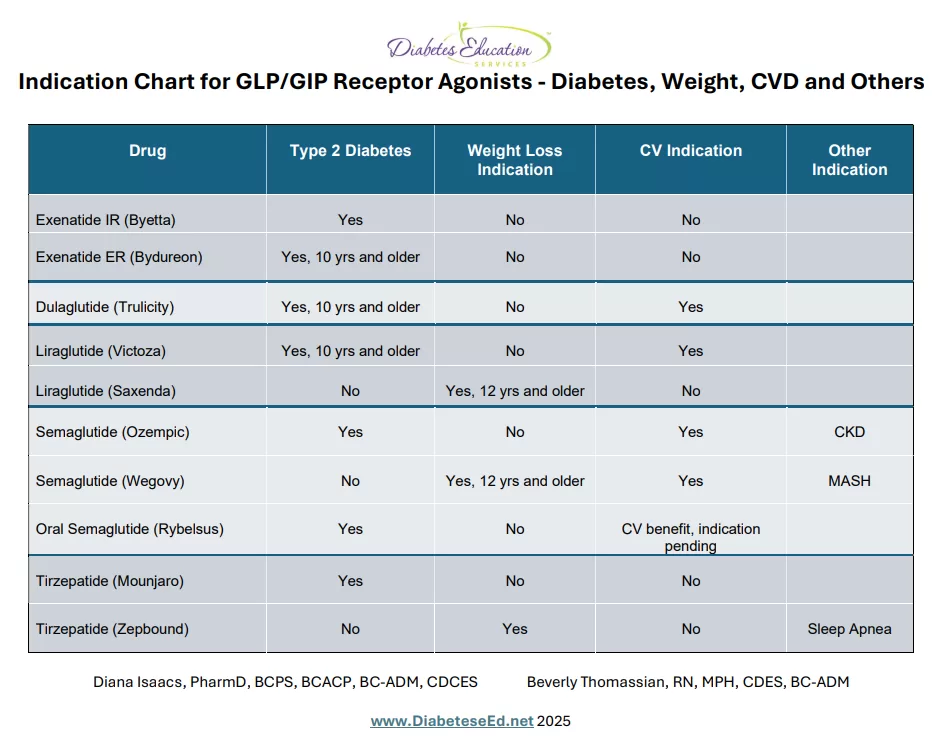
GLP-1 receptor agonists and GLP-1/GIP combination therapies have become foundational treatments for type 2 diabetes and obesity. Their benefits now extend well beyond glycemic control and weight loss, with growing evidence supporting their use in the management of steatosis liver disease, obstructive sleep apnea, cardiovascular disease, and chronic kidney disease. Emerging studies even suggest potential therapeutic benefits in heart failure, further expanding the clinical utility of this powerful drug class.
Summary Table to Keep Track of Indications for GLP-GIP RA’s
We are excited to share this summary sheet of all the current indications for the incretin class of medication over the past ten years. This article provides a brief summary of all the indications plus you can refer to our newly published GLP-GIP RA Indication Cheat Sheet here. We created this summary so busy healthcare professionals (YOU) could keep track of all these evolving developments. Keep checking back, because we will keep updating this chart based on the lasts research findings and FDA Approvals.
✔️ Approval for Diabetes and Weight Management
Over the past decade, three GLP-1/GIP receptor agonists—liraglutide, semaglutide, and tirzepatide—initially approved by the FDA for adults with type 2 diabetes, have also gained FDA approval for chronic weight management in people without diabetes, provided they meet specific criteria: a BMI of ≥30, or ≥27 with at least one weight-related condition such as hypertension, dyslipidemia, or type 2 diabetes.
✔️ Expanded Approval for Pediatrics
With rising rates of diabetes and excess weight in children, the FDA has expanded the use of incretin-based therapies to younger populations. Three diabetes incretin medications are now approved for pediatric use starting at age 10 (see cheat sheet), and two incretin-based weight management medications are approved for children aged 12 and older.
✔️ Approval for Cardiovascular Risk Reduction
Ongoing cardiovascular outcomes trials (CVOT) studies clearly demonstrated that three of the diabetes incretin medications (liraglutide, dulaglutide and semaglutide) and one of the weight loss versions of this class (Wegovy) also reduce cardiovascular disease risk. The CVOT results for the oral version of semaglutide (Rybelsus) are pending, with results expected soon.
✔️ Approval for Sleep Apnea, MASH, CKD
Tirzepatide (Zepbound) — originally approved for weight loss — now also holds FDA approval for the treatment of moderate to severe obstructive sleep apnea in adults with obesity. Semaglutide (Wegovy) has been granted FDA approval for the treatment of metabolic-associated steatohepatitis (MASH, or NASH with fibrosis). Meanwhile, semaglutide (Ozempic) is now FDA‑approved for reducing the risk of kidney disease progression and renal outcomes in people with type 2 diabetes and chronic kidney disease. And there is more to come!
Looking toward the future
The wheels of progress on multi-agonists, oral small molecules, and tissue-targeted approaches are moving forward at breakneck speed.
This brilliant JAMA review by Gonzalez-Rellan & Drucker — one of the original discoverers of GLP-1 biology. In a single piece, he concisely captures:
– the state of the field
– the most important clinical trials
– where the pipeline is headed
These Clinical trials are raising the bar and expanding future treatment options:
• Retatrutide → ~24% weight loss (triple agonist)
• MariTide → 12–16% weight loss (GLP-1 + GIP antagonist)
• CagriSema → up to 20% weight loss + ~2% HbA1c drop
• Orforglipron → oral GLP-1, HbA1c –1.5%, weight –7.6%
• IcoSema → weekly semaglutide + once-weekly insulin
Even more exciting? There are clinical trials evaluating if these GLP-1 and multi-agonists can address neurodegeneration, addiction, inflammation, and beyond.
Access, Cost and Shifting Landscape
As people on these agents meet their weight and diabetes goals, there is concern that insurance companies will reduce or stop coverage given the high price tag of lifelong incretin therapy. Hopefully, competition may drive price pressure or encourage development of biosimilars/generics in the long term. In addition, as new agents enter the market, payer coverage and prior-authorization landscapes will shift—especially with expanded medication benefits.
Diabetes Healthcare Professionals Consider Whole Person
As diabetes healthcare professionals, we want to carefully consider which medication best matches the people in our care, considering individual values and preferences along with co-conditions including diabetes, cardiorenal metabolic co-conditions, MASH, sleep apnea and weight goals.
Download Summary Sheet Here!
Join us live in San Diego Next Week

It’s not too late to register!
Get ready for certification & take your practice to the next level
October 22nd – 23rd, 2025
DiabetesEd Training Seminar
30+ CEs with Expanded Accreditation!

Join our expert team for engaging, interactive sessions that bring the ADA Standards of Care to life—covering medications, behavior change, technology, and more. Ideal for CDCES or BC-ADM exam prep, this course also includes a 4-hour Virtual Medical Nutrition Therapy Toolkit and bonus content that also meets CDCES renewal requirements.
Program Objectives:
Upon completion of this activity, participants should be able to:
- Describe the current ADA Standards for diagnosis, goals, and person-centered diabetes management across the lifespan.
- Demonstrate insulin pattern management and dosing strategies in clinical scenarios.
- Implement timely screening and risk reduction strategies for microvascular and cardiovascular complications.
- Incorporate behavior change techniques and medical nutrition therapy to support people with diabetes self-management and lifestyle adjustment.
Expert Faculty:

Diana Isaacs, PharmD, BCPS, BCACP, CDCES, BC-ADM, FADCES, FCCPCES

Beverly Thomassian, RN, MPH, CDCES, BC-ADM
The use of DES products does not guarantee the successful passage of the certification exam. CBDCE and ADCES do not endorse any preparatory or review materials for the CDCES or BC-ADM exams, except for those published by CBDCE & ADCES.