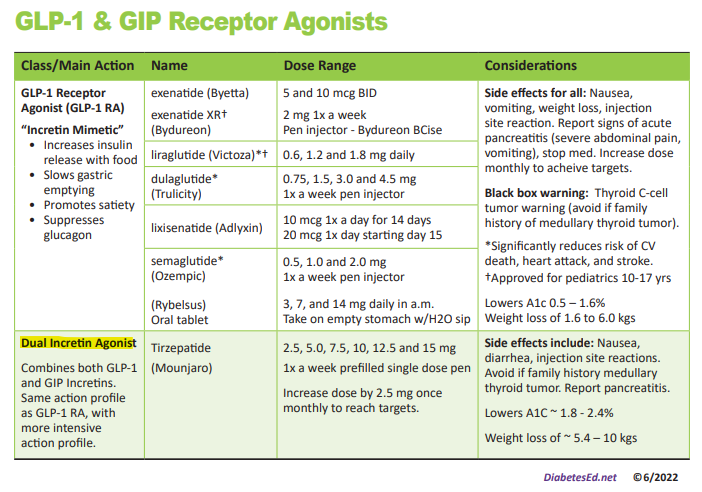
We have just added this novel, first in class, dual incretin hormone therapy, Tirzepatide (Mounjaro), to our printed version of our Diabetes Medication PocketCard.
This new twin therapy includes not only a GLP-1 Receptor Agonist, but also a Glucose-dependent insulinotropic polypeptide (GIP), which magnifies the therapeutic effectiveness. The SURPASS studies indicate that study participants experienced an A1C drop of up to 2.5% and weight loss of up to 10kg or more.
How does it work?
Incretins (GLP-1 and GIPs) play a major role in glucose regulation post prandially. Incretins are gut hormones that stimulate insulin release from the pancreas when glucose rises in response to food ingestion. They keep blood sugars in check as well as activating the satiety center, to increase the sense of fullness. Incretins slow gastric emptying and also curb post-meal glucagon release, decreasing post prandial glucose spikes. Unfortunately, people with type 2 diabetes, make less than half of the usual amount of the GLP-1 and GIP hormones, which contributes to chronically elevated glucose levels.
To date, therapies that have only included the Glucagon-Like Peptide-1 (GLP-1) have demonstrated major success in managing Type 2 DM.
By creating a formulation with BOTH the GIP and GLP incretin hormones, the A1C lowering and weight loss effectiveness has been significantly amplified.
First in Class – Dual Incretin Injectable Therapy
Tirzepatide is a novel synthetic peptide, engineered to provide a once-weekly injectable medication that acts on glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors.
Tirzepatide has been granted FDA approval for treatment of type 2 diabetes based primarily on a series of trials known as SURPASS. The SURPASS 1 trial compared increasing doses to evaluate dose response. The SURPASS 2-5 Trials compared tirzepatide to the GLP-1 semaglutide and to the basal insulins degludec and glargine. In all of the trials, the findings were impressive.
Summary of SURPASS Trials and Results based on Tirzepatide Package Insert.
- SURPASS -1 Dose efficacy study ranging rom 5 to 15 mg, evaluating A1C and weight loss. Up to 85% of participants on tirzepatide achieved an A1C of less than 7%. A1C drop of 1.7% with 15mg dose. Weight loss of up to 7.8kgs.
- SURPASS-2 Compared tirzepatide with patients on metformin to semaglutide. Up to 86% of participants on tirzepatide achieved an A1C of less than 7%. A1C drop of 2.3% with 15mg dose. Weight loss of up to 11.2 kgs.
- SURPASS-3 Compared tirzepatide with patients on metformin, with our without an SGLT-2 Inhibitor to Insulin degludec. Up to 83% of participants on tirzepatide achieved an A1C of less than 7%. A1C drop of 2.1% with 15mg dose and weight loss of up to 11.3kgs.
- SURPASS-4 Compared tirzepatide with people on metformin, and/or sulfonylureas and/or SGLT-2s with Insulin Glargine in people with increased cardiovascular risk. Up to 85% of participants on tirzepatide achieved an A1C of less than 7%. A1C drop of 2.4% with 15mg dose and weight loss of up to 10.6 kgs.
- SURPASS-5 Compared tirzepatide vs placebo in people taking Insulin glargine with or without metformin. Up to 90% of participants on tirzepatide achieved an A1C of less than 7%. A1C drop of 2.4% with 10mg dose and weight loss of up to 10.5kgs.
Tirzepatide (Mounjaro) Indications and Dosing
This once a week injectable is for adults with type 2 diabetes as an adjunct to diet and exercise to improve glycemic control and support weight loss. Tirzepatide is delivered with a single dose prefilled pen.
Starting Dose: 2.5 mg SC every week for 4 weeks initially; THEN increase to 5 mg SC every week. If additional glycemic improvement is needed, increase by 2.5-mg increments after at least 4 weeks at current dose.
Note: The initial 2.5-mg dose is intended for treatment initiation and is not effective for glycemic control
Maximum dose: 15 mg SC qWeek
Common Adverse Side effects: Nausea, diarrhea, abdominal discomfort and injection site hypersensitivity reactions. There is also a black box warning to avoid if family history of medullary thyroid tumor and to report any signs of pancreatitis immediately.
Cost: Tirzepatide is comparably priced to other GLP-1 RAs on the market at $974.33 for four weekly doses, regardless of dose size. Hopefully, insurance companies will recognize the long term benefits of these newer classes of agents, and increase coverage of these beneficial treatment options.
In conclusion: This new medication class substantially lowers blood sugars and body weight, with a once a week injection. Coupled with healthy eating and keeping active, people with diabetes have the opportunity to improve their health and quality of life with this novel medication class.
Author’s note: Beverly Thomassian has no conflict of interest to report and distilled the content of this article from the SURPASS study results and the tirzepatide package insert.