Xeris Pharmaceuticals new pre-filled glucagon pen, Gvoke, just received FDA’s stamp of approval to treat severely low blood sugar levels in people with diabetes.
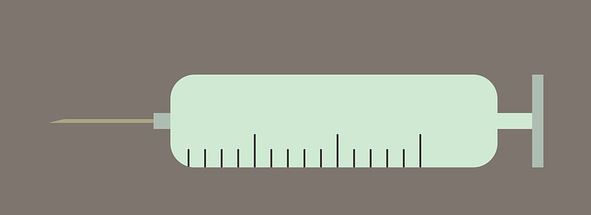
The Gvoke glucagon pen is filled with a liquid stable form of glucagon and is approved for use in people with diabetes, age 2 and above. Xeris will have the pre-filled syringe version available in 4-6 weeks and the auto-injector version available in 2020.
The Xeris glucagon pen was created to simplify glucagon injections. This new Gvoke pen does not require any mixing, since it is filled with liquid stable glucagon.
Availability of a glucagon delivery device is critical to treat severe hypoglycemia and prevent serious consequences of untreated severe hypoglycemia such as cardiovascular events, seizure, coma, or even death.
Read more about the Gvoke Glucagon Pen and auto-injector here .
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]








