For last week’s practice question, we quizzed test takers on the best action before starting Tirzepatide (Mounjaro). 67% of respondents chose the best answer. We want to clarify and share this important information, so you can pass it on to people living with diabetes and your colleagues, plus prepare for exam success!
Before we start though, if you don’t want any spoilers and haven’t tried the question yet, you can answer it below: Answer Question
Question:
AR is 36 years old with type 2 diabetes and a BMI of over 40. Current A1C 7.9%, UACR less than 30, and GFR more than 60. Current diabetes medications include metformin, sitagliptin, and empagliflozin at maximum doses. AR is prescribed the new dual incretin tirzepatide (Mounjaro) to help improve glucose levels and support weight loss.
Before starting tirzepatide (Mounjaro), what action do you recommend to the provider?
Answer Choices:
- Repeat the UACR and GFR to verify kidney function.
- Stop the sitagliptin.
- Decrease metformin dose to prevent hypoglycemia.
- Evaluate thyroid function.
Getting to the Best Answer
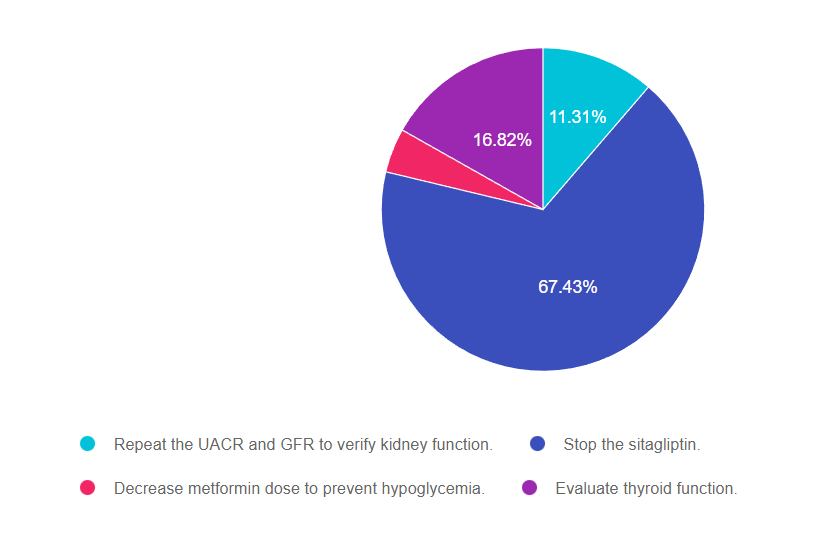
Answer 1 is incorrect. 11.31% chose this answer, “Repeat the UACR and GFR to verify kidney function.” This is not the best answer because JR has great kidney function, evidenced by a normal UACR and GFR. So we do not need to recheck kidney function before starting this new dual incretin tirzepatide (Mounjaro).
Answer 2 is correct. 67.43% of you chose this answer, “Stop the sitagliptin.” YES, this is the best answer. The American Diabetes Association (ADA) and American Association of Clinical Endocrinologists recommend against combination therapy with a DPP4-inhibitor and a GLP-1 RA due to the lack of evidence that this strategy is beneficial. Initial research combining the two classes, reflect a negligible glucose lowering effect when GLP-1’s and DPP-4’s are combined. Given the approximate average wholesale price for the DPP-4 inhibitor of $434 and GLP-1 RA of $887, using both simultaneously is not cost effective or clinically beneficial.
Answer 3 is incorrect. 4.4.% of respondents chose this answer, “Decrease metformin dose to prevent hypoglycemia.” No need to decrease the metformin dose, since JR is not on any medication class that will cause hypoglycemia.
Finally, Answer 4 is incorrect. 16.82% chose this answer, “Evaluate thyroid function.” This class of medication is not recommended for any person with a history of medullary thyroid tumor. However, we don’t need to draw a TSH to see if this medication is safe, we would simply do a careful history to see if there is a family history of medullary thyroid tumor.
Thank you so much for reading this “Rationale of the Week”. You can download our Medication PocketCard below, for more information.
New Injectable – “TwinCretin” on Printed PocketCards
We have just added this novel, first in class, dual incretin hormone therapy, Tirzepatide (Mounjaro), to our printed version of our Diabetes Medication PocketCard.
This new twin therapy includes not only a GLP-1 Receptor Agonist, but also a Glucose-dependent insulinotropic polypeptide (GIP), which magnifies the therapeutic effectiveness. The SURPASS studies indicate that study participants experienced an A1C drop of up to 2.5% and weight loss of up to 10kg or more.








