With the approval of Tzield (teplizumab-mzwv), an anti-CD3 monoclonal antibody, we can now offer children 8 years and older an opportunity to delay the progression to type 1 diabetes for up to two years or longer and improve beta cell function.
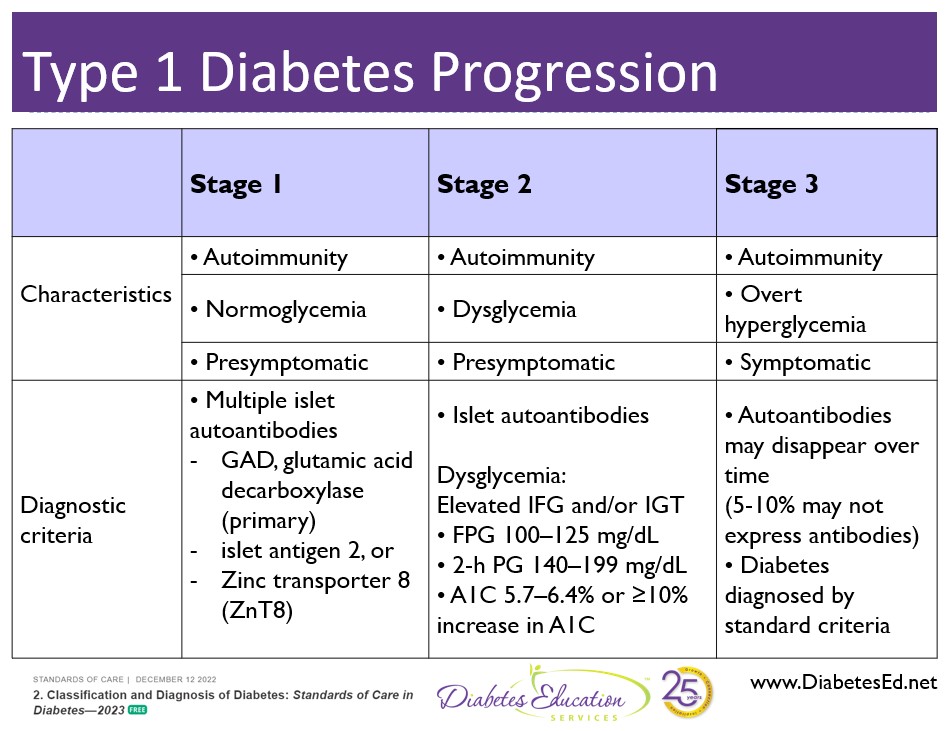
Teplizumab is the first disease-modifying therapy that impedes the progression of type 1 diabetes by binding to the surface of T cells to dampen the unwanted immune system response. It can delay the onset of symptomatic stage 3 type 1 diabetes in adults and children 8 years and older with stage 2 type 1 diabetes (see staging chart).
It is administered by intravenous infusion once daily for 14 consecutive days and is expected to cost in the region of $200,000 for the course of treatment.
Teplizumab is indicated for the individual in stage 2 type 1 diabetes, or for those with two or more islet autoantibodies and abnormal glycemia but still asymptomatic. People with stage 2 type 1 diabetes, have a nearly 100% lifetime risk of progression to clinical (stage 3) type 1 diabetes and a 75% risk of developing the condition within 5 years.
Results from a landmark clinical type 1 prevention trial conducted by TrialNet showed that with a two-week course of teplizumab:
- 72% of people in the control group developed clinical diabetes, compared to only 43% of the teplizumab group.
- The median time for people in the control group to develop clinical diabetes (stage 3) was just over 24 months, while those in the treatment group had an average of 48 months before progressing to clinical diabetes (stage 3).
Insulin production in response to glucose was impaired in the participants before they enrolled in the trial. In participants given an inactive placebo, insulin production declined through the first six months of the study. But in those treated with teplizumab, insulin production improved after treatment.
These results suggest that a single course of Teplizumab may provide long-lasting improvement in beta cell function. This, in turn, could delay or even prevent the development of type 1 diabetes in high-risk individuals.
More Screening for Type 1 Needed?
The ability to halt the progression of stage 2 type 1 diabetes from progressing to symptomatic stage 3 diabetes brings the prospect of more wide spread screening for type 1 into sharper focus. Currently, 40-50% of new type 1 diabetes is diagnosed when the individual is admitted to the hospital in a life-threatening hyperglycemic crisis or DKA. Studies suggest, identifying children at risk of future type 1 could avert DKA presentation, saving hundreds of thousands of dollars in hospital costs and allowing for early CD3 monoclonal antibody intervention.
Based on data from Trialnet, even though type 1 diabetes runs in families, 85 – 90% of people don’t have a first-degree relative with type 1 diabetes. That means, broader screening initiatives will be needed to identify those at risk for type 1 and eligible for the benefits of Teplizumab therapy.
Universal genetic or antibody screening in the future?
The Juvenile Diabetes Research Foundation is working toward a two-pronged screening approach.
This includes testing infants at birth with a genetic risk score combined with antibody screening. With this approach, providers can monitor kids with high genetic risk scores – based on human leukocyte antigen haplotypes, and provide education to family members regarding signs of hyperglycemia. In addition, antibody screening can help identify the stage of autoimmunity and help guide treatment action.
Researchers now have solid proof that immunotherapy can slow the progression of type 1 diabetes. This offers hope to conduct additional studies to extend the benefits of teplizumab, while continuing to test other immune therapies. Keep posted!
Virtual DiabetesEd Training Conference

Whether you are new to diabetes or a seasoned expert, you’ll benefit from this virtual conference with the latest research plus critical content that you can immediately apply to your clinical practice.
If you are seeking a state-of-the-art review of current diabetes care, this course is for you. Our team has been fine-tuning this course for over fifteen years, and we know what you need. This program can also be a great addition to your CDCES or BC-ADM exam study plan.
Group discounts are available!*
Download Course Flyer | Download Schedule
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.




