The Food and Drug Administration (FDA) just approved another GLP-1 RA for use in children ages 10-17 with diabetes.
The injectable extended-release exenatide (Bydureon) is now the second glucagonlike peptide-1 receptor agonist (GLP-1 RA) approved for use in pediatric type 2 diabetes and the first with once-weekly administration. Liraglutide (Victoza) a daily injectable GLP-1 RA received approval for pediatric use in 2019 and metformin an oral biguanide has long been FDA approved for use children ten and older with type 2 diabetes.
During the annual scientific sessions of the American Diabetes Association, speakers expressed alarm about the rise in youth developing type 2 diabetes, noting that the condition typically progresses more rapidly and is less likely to respond well to metformin, compared with adults.
The approval was based on a 24-week, double-blind, placebo-controlled study in 82 children with type 2 diabetes aged 10 and older. They were randomized to 2 mg once-weekly exenatide extended release or placebo. At week 24, hemoglobin A1c in those randomized to the drug had dropped by 0.25 percentage points, compared with a 0.45 percentage point increase in the placebo group.
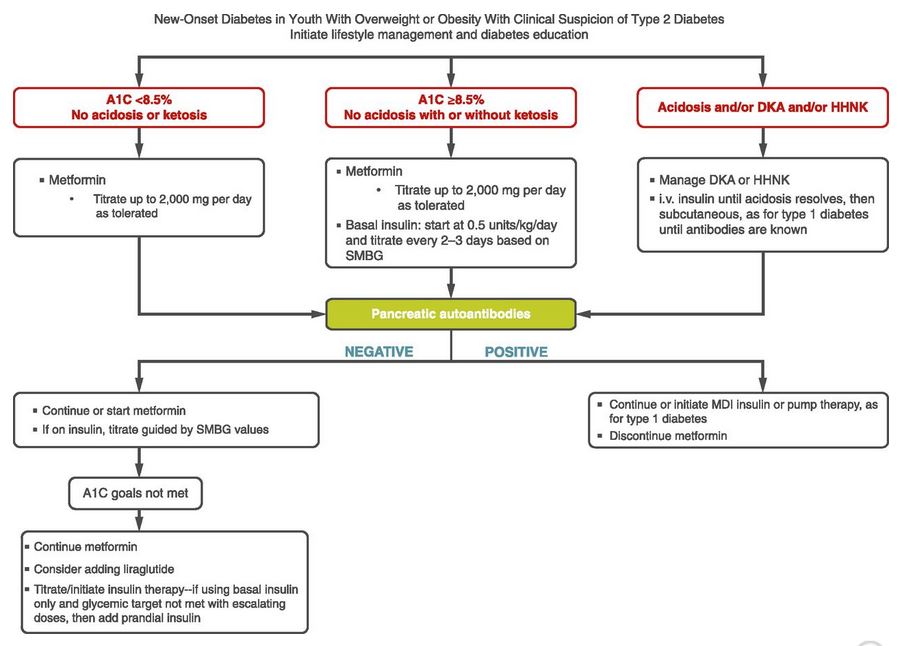
Based on the ADA Standards of Care for treating pediatrics with diabetes, the first treatment includes weight loss and exercise plus first line medications metformin and insulin if A1c is 8.5% or greater.
Furthermore, the initiation of any GLP-1 RA is only recommended once pancreatic antibodies are determined to be absent and if A1c goals have not met despite the initiation of metformin or insulin (see ADA standards algorithm below stating consider adding liraglutide (GLP-1 RA))
We have updated the electronic version of our Injectable PocketCard to reflect this update.
Download our Medication PocketCards today on our Website PocketCards , or CDCES Coach App (free).
Want to learn more about this topic and more?
Enroll in our Virtual DiabetesEd Specialist Program!
Did you miss the live conference? No worries! You can register now to watch on-demand
Whether you are new to diabetes or a seasoned expert, you’ll benefit from this virtual conference with the latest research plus critical content that you can immediately apply to your clinical practice.
If you are seeking a state-of-the-art review of current diabetes care, this course is for you. Our team has been fine-tuning this course for over fifteen years, and we know what you need. This program can also be a great addition to your CDCES or BC-ADM exam study plan.
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
In addition to informative lectures, we also use group activities and case studies to highlight the essential knowledge, skills, and strategies needed to succeed in diabetes education today!

CEs: Includes over 30 CEs
Program Info: 2021 Diabetes Educator Course Flyer & Schedule (subject to change)
Speakers: View Conference Faculty.
Dates: Your registration fee includes access to FREE podcast and all recorded webinars for one year.
Two Registration Options
Virtual DiabetesEd Specialist Conference Deluxe | Oct. 6-8 | 30+ CEs
Deluxe Virtual Program for $459 includes:
- Presentations by our team of experts
- Q & A Session with the instructor after each webinar
- State-of-the-art review of current diabetes care and technology.
- Resources for each session
- Access to FREE podcast and recorded webinars within a week of each live session for one full year.
+Plus Syllabus, Standards and Swag:
- Diabetes Educator Course 2021 Syllabus Hard Copy – over 100 pages -This spiral-bound workbook contains the printed version of all of the instructor’s slides.
- DiabetesEd Services highlighters, Medication PocketCard and Pen
2021 Diabetes Educator Course Flyer & Schedule (subject to change)
Virtual DiabetesEd Specialist Conference Basic | Oct. 6-8 | 30+ CEs
Basic virtual program for $359 includes:
- Presentations by our team of experts
- Q & A Session with the instructor after each webinar
- State-of-the-art review of current diabetes care and technology.
- Resources for each session
- Access to FREE podcast and recorded webinars within a week of each live session for one full year.
2021 Diabetes Educator Course Flyer & Schedule (subject to change)
Team of Experts: Our team of expert faculty has been fine-tuning this course for over fifteen years and we know what you need to succeed! In addition to informative lectures, we also use group activities and case studies to highlight the essential knowledge, skills, and strategies needed to succeed in diabetes education today!
Bonus Courses worth 12+ CEs, FREE
When you register for our Virtual Course, you have immediate access to these Bonus DiabetesEd University Online Courses – for FREE!
- Test Taking Toolkit – Over 200 sample test questions!
- Level 2 – Assessing and Promoting Well-Being: From Population Health to a Person-Centered Approach 1.5 CEs
- Level 2 – Hospital and Hyperglycemia 1.5 CEs
- Level 2 – Hyperglycemic Crisis, DKA and HHS Standards 1.0 CEs
- Level 2 – Meds Management Update for Type 2 – 1.5 CEs
- Level 2 – Setting up a Successful Diabetes Program 1.5 CEs
- Level 2 – Pregnancy and Diabetes 1.5 CEs
- Level 2 – From Tots to Teens – Diabetes Standards 1.5 CEs
- Level 2 – Older Adults and Diabetes 1.5 CEs
- Mindfulness and Compassion in the Diabetes Encounter – 1.0 CE
2021 Diabetes Educator Course Flyer & Schedule (subject to change)
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Accreditation: Diabetes Education Services is an approved provider by the California Board of Registered Nursing, Provider 12640, and Commission on Dietetic Registration (CDR), Provider DI002. Since these programs are approved by the CDR it satisfies the CE requirements for the CDCES regardless of your profession.*
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.









