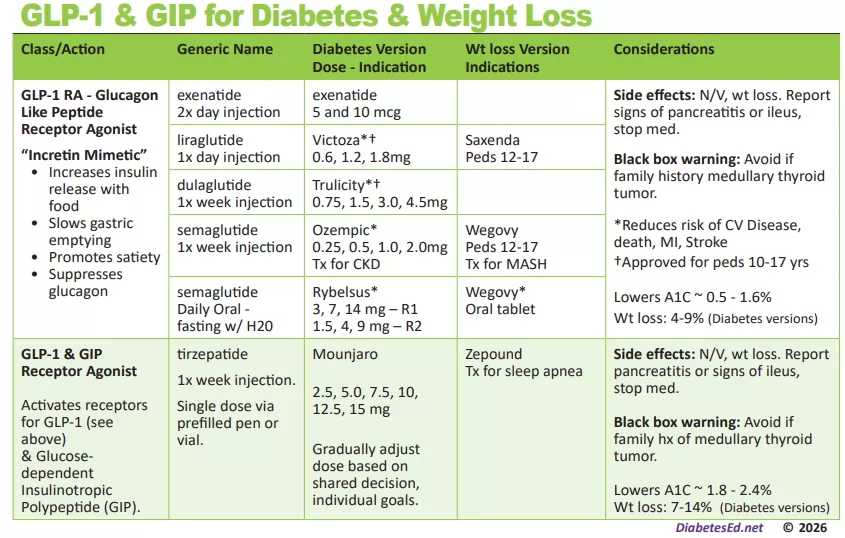
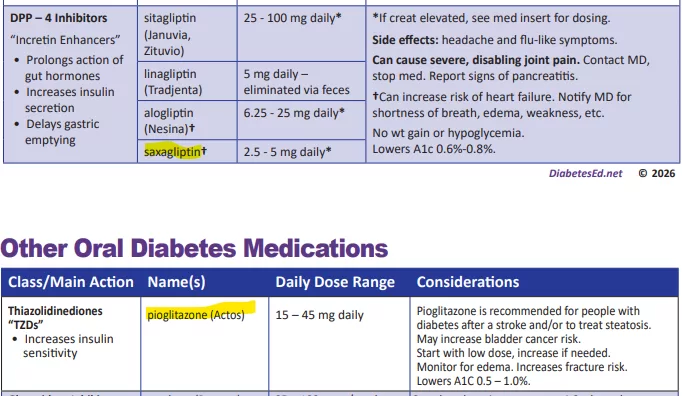
Download Free Diabetes Cheat Sheets!
Meds PocketCard Refresh for 2026!
Tirzepatide approved for weight loss
The U.S. Food and Drug Administration approved tirzepatide (Zepbound) injection for chronic weight management in adults with a BMI of 30 or with a BMI of 27 or greater with at least one weight-related condition (such as high blood pressure, type 2 diabetes, or high cholesterol) this November. Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist.
Up to 25% body weight loss
The SURMOUNT clinical trials demonstrated that tirzepatide therapy not only improved glucose levels but also had a substantial impact on body weight reduction. At the highest dose (15 mg), people taking Zepbound lost on average 48 lb., while at the lowest dose (5 mg), people lost on average 34 lb. (compared to 7 lb. on placebo).
Additionally, 1 in 3 clinical participants taking Zepbound at the highest dose lost over 58 lb. (25% of body weight), compared to 1.5% on placebo, according to data not controlled for type 1 error. The average starting weight was 231 lb. Zepbound is recommended along with healthy eating and increased activity.
About the SURMOUNT clinical trial program
The SURMOUNT phase 3 global clinical development program for tirzepatide in chronic weight management began in late 2019 and has enrolled more than 5,000 people with obesity or overweight across six registration studies, four of which are global. SURMOUNT-1 and SURMOUNT-2 were submitted to the FDA and demonstrated tirzepatide significantly reduced body weight compared with placebo in people living with obesity or overweight, with or without type 2 diabetes.
The dual action of tirzepatide on both GIP and GLP-1 receptors allows for a comprehensive approach to blood sugar regulation with substantial weight loss. Tirzepatide, the active ingredient in Zepbound, is already approved under the trade name Mounjaro to be used along with diet and exercise to help improve blood sugar in adults with type 2 diabetes. With this new FDA approval for weight loss, people who meet the BMI and risk criteria qualify to use this novel medication class, whether or not they have diabetes.
Cost
Zepbound is expected to be available in the U.S. by the end of the year in six doses (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg) at a list price of $1,059.87
Side effects:
Zepbound may be associated with gastrointestinal adverse reactions, sometimes severe. The most commonly reported adverse events (observed in ? 5% of clinical trial participants) were nausea, diarrhea, vomiting, constipation, abdominal pain, dyspepsia, injection-site reactions, fatigue, hypersensitivity reactions, eructation, hair loss, and gastroesophageal reflux disease. In studies, most nausea, diarrhea, and vomiting occurred when people increased their dose – but the effects generally decreased over time. Zepbound may cause tumors in the thyroid, including thyroid cancer. Watch for possible symptoms, such as a lump or swelling in the neck, hoarseness, trouble swallowing, or shortness of breath, and avoid using if there is a family history of medullary thyroid carcinoma (MTC).
For more information, see FDA Approval Information.
Want to learn more about Diabetes Medications?
Meds Management Update for Type 2 Diabetes
Airs live on December 21, 2023, at 11:30 am PST

Topics:
- Describe the role of Diabetes Care & Education Specialists in advocating for optimal therapeutic approaches.
- Discuss the application of the new ADA/EASD Guidelines to improve glucose and reduce CV and renal risk.
- List strategies to initiate & adjust oral & injectable therapy using a person-centered approach.
In this exciting webinar, Coach Beverly walks participants through the “Management of Hyperglycemia in Type 2 Diabetes” as outlined by the most recent American Diabetes Association (ADA) guidelines. She kicks-it off with a brief overview of the different classes of medications then uses a case study approach to apply the ADA algorithm.
Can’t make it live? No worries. We will send post the recorded version to the Online University within 24 hours of the broadcast
Instructor: Beverly Thomassian RN, MPH, CDCES, has been Board Certified in Advanced Diabetes Management for over 20 years. She is an Associate Clinical Professor at UCSF, a working educator, and a nationally recognized diabetes expert. She has a Master’s Degree in Public Health from UCLA, with a focus on behavioral health and education.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
Diabetes Drugs & Medicare Negotiations | Diana Issacs, PharmD Breaks it Down

Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES is a thought leader in the field of diabetes and has the opportunity to share her expertise as a podcast host, along with colleague Natalie Bellini, DNP, as part of HCP Live and Diabetes Dialogue’s educational series. In a recent program episode, “What Medicare Drug Price Negotiations Mean for Diabetes”, Dr. Isaacs and Dr. Bellini provide an informative and lively discussion on the newly announced Medicare Medication Price negotiation program.
What is this Medicare Drug Hubbub all about?
In order to improve the affordability of medications for those 65 and older with Medicare Part D, the Centers for Medicare & Medicaid Services (CMS), announced the first 10 drugs covered under Medicare Part D selected for negotiation as part of the Inflation Reduction Act,
The drugs chosen include the 10 drugs with the highest gross annual spending total, accounting for $50.5 billion in total Part D gross covered prescription costs per year and more than $3.4 billion in out-of-pocket costs.
Diabetes Medications Included:
There are four diabetes medications included among the ten drugs named for price reductions in this first go-round, which if approved, would take effect in January 2026.
The diabetes agents chosen for negotiation include:
- The SGLT’s empagliflozin (Jardiance) and dapagliflozin (Farxiga)
- An DPP-IV sitagliptin (Januvia)
- and Novo Nordisk-specific insulin aspart products.
In the podcast, Dr. Isaacs and Dr. Bellini, comment on the lack of any GLP-1 or GLP-1 /GIP combo medications in the initial list and are curious about the inclusion of two SGLT-2 inhibitors and no basal insulin. In addition, there was some head-scratching around including insulin in the negotiations, since there is already a $35 Medicare payment cap on insulin therapy.
This group of medications was chosen in part, because according to CMS statistics, these agents accounted for more than $16 billion in total Part D spending from June 1, 2022, through May 31, 2023, which served as the evaluation period for the decision-making process.
As far as when this program will start, negotiated prices for the selected drugs will be announced by September 1, 2024, and those prices will come into effect starting January 1, 2026. The long-term goal is to expand this program as part of the Inflation Reduction Act. By 2027, the goal is to have 15 more medications covered under Part D for negotiation and up to 15 more drugs for negotiation in 2028, including drugs covered under Part B and Part D, and up to 20 more drugs for each year after.
Expect push backs from pharmaceutical companies. Drugmakers have said the new provisions are unconstitutional and have filed a series of lawsuits to try to stop them.
Thank you to hosts Diana Isaacs, PharmD, an endocrine clinical pharmacist, director of Education and Training in Diabetes Technology, and codirector of Endocrine Disorders in Pregnancy at the Cleveland Clinic, and Natalie Bellini, DNP, program director of Diabetes Technology at University Hospitals Diabetes. Click this link to listen to the complete program episode, “What Medicare Drug Price Negotiations Mean
for Diabetes”.
Diana Issacs Speaks for 2 Days in San Diego at our DiabetesEd Specialist Conference – You are invited
If you want to meet Diana in person and benefit from her expert knowledge, she will be co-teaching at our three-day DiabetesEd Specialist Training Program in San Diego on October 11-13th. You won’t want to miss her ADA Standards of Care update, along with her expertise on diabetes medications and her hands-on diabetes technology explanations.
Join Dr. Isaacs & team LIVE in San Diego for our
DiabetesEd Training Conference
October 11-13th, 2023

Two Registration Options
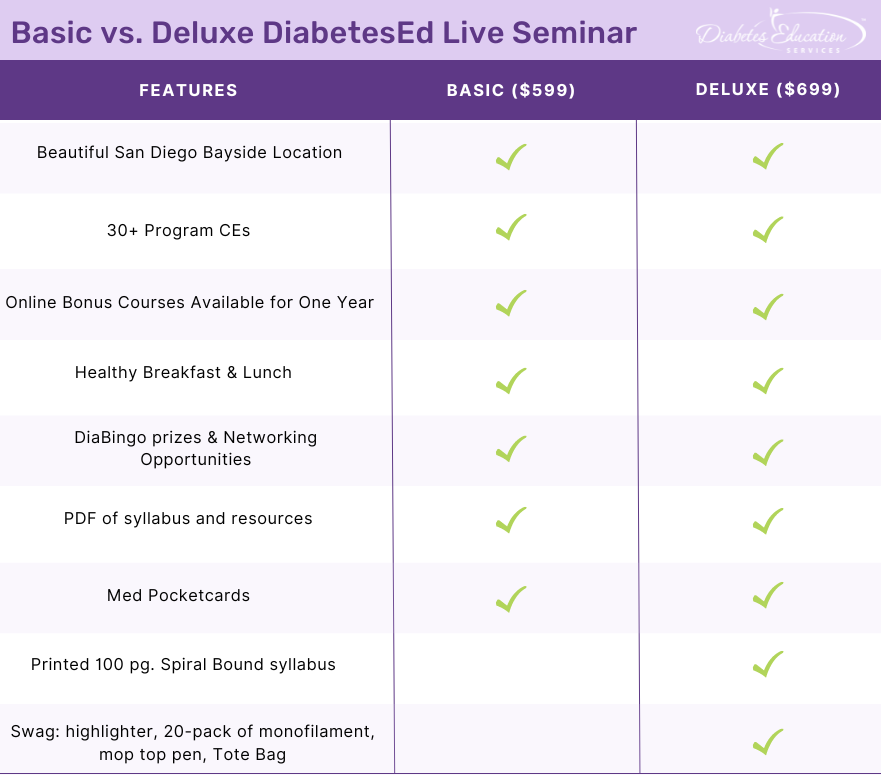
Join Coach Beverly and Team for two and a half days of knowledge-sharing, fun, networking, games with prizes, and “aha” moments in beautiful San Diego.
You don’t want to miss this one-of-a-kind learning opportunity. Get away from all those daily responsibilities and immerse yourself in a fun and intensive conference with plenty of networking opportunities.
Attendees will leave this conference with new tools and a more complete understanding of the latest advances in diabetes care, from medications to technology to Medical Nutrition Therapy!
Bring your colleagues and enjoy our friend discount.
Our team expertly translates the complex science of diabetes into understandable terms while keeping it real, practical, and fun.
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
Sulfonylurea Sold as “Street Valium”
At the recent gathering of the American Association of Clinical Endocrinology meeting, a provider shared a surprising case study of life-threatening hypoglycemia in a 33-year-old without diabetes.

Unknowingly, this individual thought they were purchasing “street Valium”, but was sold the potent sulfonylurea, glyburide, instead. As a result, their blood sugar dropped to 18 mg/dL, causing unconsciousness and the need for emergent medical assistance. This individual had purchased two unmarked, light blue pills on the street, which they thought were Valiums but turned out to be glyburide. Since sulfonylureas aren’t detected in urine toxicology screens, the symptoms of hypoglycemia may be mistakenly attributed to other causes or drugs. The only way to detect the presence of sulfonylureas is through blood tests.
“Physicians should be aware of this possibility and consider intentional or unintentional sulfonylurea abuse, with or without other drugs,” Amanda McKenna, MD, a first-year endocrinology fellow at the Mayo Clinic, Jacksonville, Florida, and colleagues say in a poster presented at the American Association of Clinical Endocrinology (AACE) Annual Meeting 2023.
Glyburide has a similar appearance to street valium. It is cheaper and easier to acquire than Valium (a controlled substance) which explains its appearance in illicit drug sales over the past two decades. However, since glyburide stimulates sustained insulin secretion, consumption can lead to life-threatening prolonged hypoglycemia. In addition, the person consuming it may attribute their symptoms to the “Valium” they thought they were taking and is vulnerable to severe hypoglycemia.
If hypoglycemia is detected, D50W is commonly used to immediately raise glucose levels. But, since sulfonylurea has a long half-life, blood sugars may plummet again. The most effective treatment to sustain blood sugars for those with glyburide toxicity is the administration of octreotide. Octreotide, a long-acting somatostatin agonist, reverses the insulin-releasing effect of sulfonylureas on pancreatic beta cells, resulting in diminished insulin secretion.
Cases like these have been reported for the past two decades. But how many incidents are we missing? Sharing this information with first responders, emergency department staff, and our communities can save lives.
Read more here
NEW: Everything Bundle
For the first time, we are offering all of our Online Courses and Training Programs in ONE bundle!
Now on sale, Enroll Now & Save $100!
40+ online courses | 85+ CEs

This exclusive Everything Bundle provides access to ALL of our Online University Courses and Training Programs. This bundle is perfect for those who need CEs to renew their license or diabetes certification or are looking for a comprehensive update on all topics of diabetes.
Subscribers enjoy over 40 courses taught by Coach Beverly and her team of experts on topics ranging from Diabetes Distress to MNT, Technology to Pattern Management with a focus on providing evidence-based, person-centered diabetes care.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
Lilly Caps Insulin at $35
Today, Lilly announced caps on out-of-pocket insulin costs at $35 per month and price reductions of 70% for its most commonly prescribed insulins.
- Effective immediately, Lilly will automatically cap out-of-pocket costs at $35 at participating retail pharmacies for people with commercial insurance using Lilly insulin.
- People who don’t have insurance can continue to go to InsulinAffordability.com and immediately download the Lilly Insulin Value Program savings card to receive Lilly insulins for $35 per month.
In addition, according to their website announcement, Lilly is reducing the list price of insulins:

- Cutting the list price of its non-branded insulin, Insulin Lispro Injection 100 units/mL, to $25 a vial, effective May 1, 2023.
- Cutting the list price of Humalog® (insulin lispro) 100 units/mL and Humulin® (insulin human) injection 100 units/mL by 70% in the fourth quarter 2023.
- Launching RezvoglarTM (insulin glargine-aglr) injection, a basal insulin that is biosimilar to, and interchangeable with, Lantus® (insulin glargine) injection, for $92 per five pack of KwikPens®
“The aggressive price cuts we’re announcing today should make a real difference for Americans with diabetes. Because these price cuts will take time for the insurance and pharmacy system to implement, we are taking the additional step to immediately cap out-of-pocket costs for patients who use Lilly insulin and are not covered by the recent Medicare Part D cap” said David A. Ricks, Lilly’s Chair and CEO.
American Diabetes Association (ADA) Celebrates
“The American Diabetes Association (ADA) is the leading voice advocating for insulin affordability and is working to ensure that all people with diabetes have access to the care they need. We applaud Eli Lilly for taking the important step to limit cost-sharing for its insulin, and we encourage other insulin manufacturers to do the same. While we have been able to help achieve significant progress on the issue of insulin affordability, including Medicare’s new out-of-pocket cost cap on insulin, state copay caps, and patient assistance developments from insulin manufacturers, we know that our work is not done. We will work to ensure that Eli Lilly’s patient assistance program is benefiting patients as intended and continue the fight so that everyone who needs insulin has access.”? — Charles “Chuck” Henderson, CEO of the American Diabetes Association
Insulin has grown increasingly expensive in recent years despite the introduction of new competition. Many people with diabetes ration their medicines or discontinue them because of the cost. More than 30 million Americans live with diabetes, and more than seven million of them rely on insulin.
Join the ADA’s Fight for Affordable Insulin!
Click here to learn more and get involved!
Medicare Caps Insulin at $35 a month
Read our blog here for more info
FREE Insulin PocketCard with Complete Insulin Listing
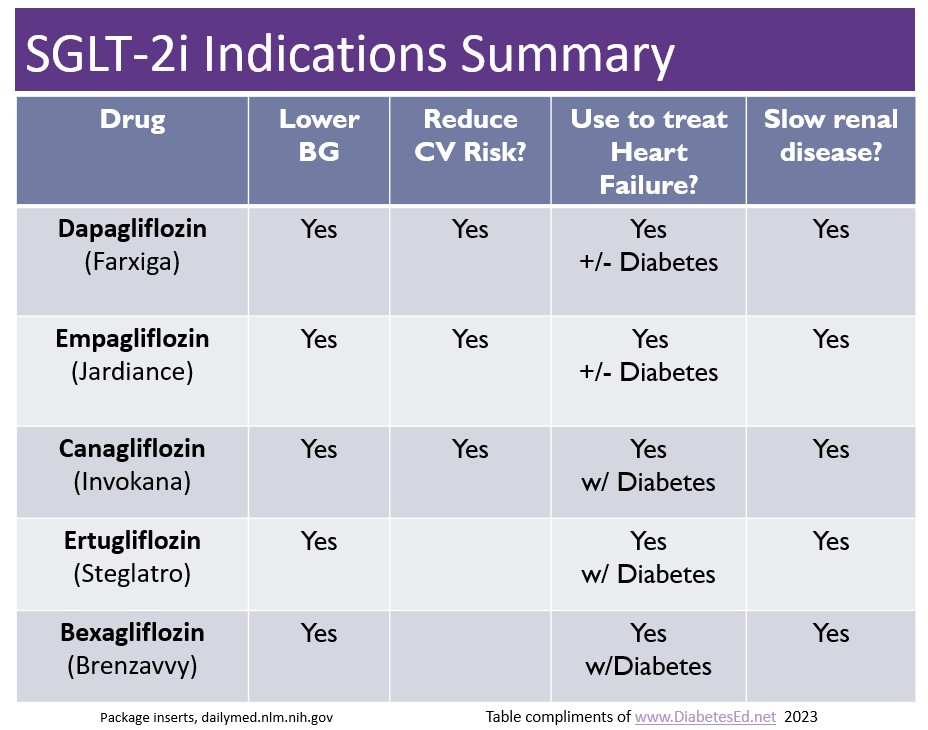
New “Zavvy” SGLT-2 Inhibitor Approved
There are now five SGLT-2 Inhibitors that are FDA approved for the treatment of diabetes. The latest one is bexagliflozin (Brenzavvy). We created the chart below to summarize the features and indications of the various SGLT-2i’s. We have also updated our eMedication PocketCard, with this 5th SGLT-2i.
According to TheracosBio, FDA approval of bexagliflozin was based on 23 studies in over 5,000 patients with type 2 diabetes. At a dose of 20mg a day, bexagliflozin decreases A1C by 0.6% to 1.0%, decreases body weight by about 3 kg and drops systolic blood pressure almost 3 mm Hg on average. This treatment can be utilized as a monotherapy or combined with metformin or as an add-on to standard-of-care treatment, including metformin, sulfonylureas, insulin, DPP-IV inhibitors or combinations of all these agents.
GFR cut off of 30: For those with chronic kidney disease, bexagliflozin was safe and well tolerated, however it is not recommended if the eGFR less than 30 mL/min/1.73 m2 and is contraindicated for those on dialysis.
No additional CV benefit data. Bexagliflozin was not found to be superior to placebo in reducing MACE (a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for unstable angina). In a trial that included patients with type 2 diabetes and either established CVD or multiple risk factors for CVD, 10.1% (57/567) of the placebo group experienced 1 MACE event vs 7.9% (89/1132) of the bexagliflozin group. However, while the phase 3 data do not indicate any apparent CVD risk, a robust CVD outcomes trial has not been done.
Lower extremity caution: An increased, although not statistically significant, incidence of lower limb amputations was observed among patients treated with bexagliflozin (8.3 vs. 5.1 events per 1,000 patient-years; HR = 1.64, 95% CI, 0.70, 3.82).
Other warnings include genital mycotic infections, DKA, volume depletion, urinary tract infections and urosepsis, similar to other SGLT-2 inhibitors. Read more here.
Download eMedication PocketCard
$35 Cap on Insulin for Medicare Recipients?
Starting in 2023, the Inflation Reduction Act, limits insulin out-of-pocket costs at $35 for a month for each insulin product covered by an individual’s Medicare Part D prescription drug plan or Medicare Advantage plan.

Under a Medicare Part D prescription drug plan, if insulin is a covered insulin product, the $35 cap for a month’s supply for each insulin product applies, beginning on January 1, 2023.
A covered insulin product is one that is included in a Part D sponsor’s formulary.
This includes any new insulin products that become available during the plan year. An insulin product might also be considered covered in other instances and there can be changes in covered products during the year.
In addition, under Medicare Part B, if insulin is delivered through a traditional pump that is covered under the durable medical equipment benefit, the cost-sharing amount for a person with Medicare is capped at $35 for a month’s supply of insulin beginning July 1, 2023.
The cap applies only to insulin products on a plan’s formulary, or the list of covered medications.
Unfortunately, most drug plans don’t cover the more than 70 insulin products on the market. And some plans actually charge less than $35 a month for particular products, but those lower copays are difficult to ferret out on the Medicare Plan Finder. Adding to the complexity of making a choice of plans, many people with diabetes use several prescription drugs besides insulin.
End result: Finding the lowest-cost, most effective plan that covers all of a participant’s medicines among the 20 or more plans that might be offered in someone’s zip code can be complicated, at least for 2023.

Strategies to succeed? Medicare officials and the local offices that support beneficiaries strongly recommend getting help. “People with Medicare who take insulin are encouraged to call 1-800-MEDICARE or to contact their State Health Insurance Assistance Programs (SHIP) for help comparing coverage and costs this year,” Meena Seshamani, M.D., director of CMS’s Center for Medicare, said in an emailed statement. This is just a one-year glitch, the Centers for Medicare & Medicaid Services says, so it’s best handled by those with practice navigating the Plan.
Experts suggest Medicare recipients make a list of all their prescriptions, including the dosages and how often they are refilled. Then go to Medicare.gov and create an account — it allows individuals to store their drug list securely, which can speed up the task of finding a plan.
Then they have two choices:
- Call 1-800-MEDICARE and seek help there, or
- Go to the SHIP website to look up the agency that administers SHIP in your state.
The best bet is to call the state agency to find the county or other local office and contact a trained counselor who can analyze plan options. SHIPs objective is to provide comprehensive, unbiased information on Medicare. The counselor will make sure, for example, that any Medicare Advantage plan you consider actually covers the insulin products you need.
Click the Links Below for More Info.
Don’t worry if you can’t make our webinars live. Your registration guarantees access to the recorded version in the Online University.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.