Free Resource Friday | 2020 (Vision Needed to View) ADA Injectables Algorithm
Happy Friday! As I get older, font sizes seem to be getting smaller. Excited to see the updated Injectables Algorithm, I turned my ADA Book to Standard 9 and quickly realized that in order to read the print, I had to hold the page inches from my nose. And there was no way could I read the footnotes even using my iphone flashlight to illuminate the words.
Thank goodness I collaborate with wonderful staff who boast youthful eyes and 2020 vision.
Robert (our Assistant Director and my high school senior) set to work recreating a draft of the document on Word. Getting the arrows to line up and fitting all the content in little boxes, was challenging, but he persevered. Taryn added the footnotes and I completed the final editing and the creation of the PDF.
We created this readable version because the algorithm is an important document that can be used to promote a collaborative practice.
Coach Beverly
I consider the ADA Standards my clinical “playbook”. The medication algorithms are of particular importance because it provides us with the opportunity to engage in a collaborative practice with our referring providers. “These are the evidence based medication recommendations from the ADA. I use it as a guide to recommend medication additions or changes”.
Enjoy our font enhanced Figure 9.2: Intensifying to Injectable Therapy
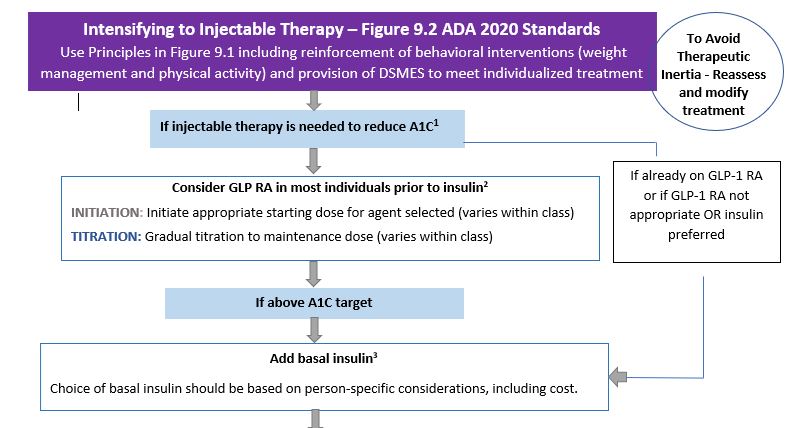
Then we can start a conversation exploring if a collaborative practice and medication adjustments using the algorithm is comfortable for the provider and perhaps include into our practice guidelines.
Enjoy this helpful 2 page reference guide, on us!
Want more Standards of Care updates?

The 2020 Standards of Care is ready for viewing. Coach Beverly highlighted changes from 2019-2020 and summarized important need-to-know content for CDCES’s!
Purchase our Level 1 Standards of Care, or buy the full Level 1 bundle to benefit from all the 2020 updates! Earn 9.0 CEs for $109
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Novo Insulin New Affordability Options
Over the past few years, providers and advocacy groups rallied to make insulin affordable for all people with diabetes.
In line with other insulin manufacturers, starting on January 2nd of this year, Novo Nordisk launched a discounted insulin program to help improve access to insulin.
My$99 Insulin Cash Card Program: By using the Cash Card Program, participants can get up to three vials or two packs for $99 of FlexPen®/FlexTouch®/PenFill® pens of any combination of Novo Nordisk Inc. insulins. For most people, this provides about a one month supply of insulin.
To benefit from this discounted rate, users need to enroll at NovoCare.com to receive an online card that can be used at their usual pharmacy, whether or not they have insurance.
Novo Nordisk human insulin is also offered at Walmart and CVS for about $25 for a 10mL vial.
Follow-on brand insulins: Follow-on brand (also called FOB or authorized generics) versions of NovoLog® and NovoLog®
Novo has cut the price by about 50% for the FOB insulins listed below.
Because these Follow-On Brands are “authorized generic,” those with a prescription for NovoLog will be able to buy these Follow-On Brands without a new prescription.
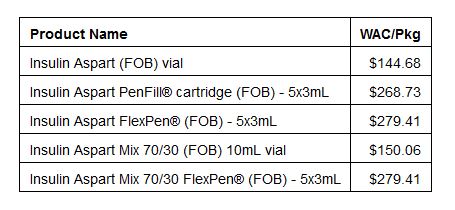
People can order them at the pharmacy and they’ll be available for pick up in 1-3 business days.
Immediate Supply Option
By calling 1.844.NOVO4ME (1.844.668.6463) or by visiting NovoCare.com, people who are struggling to access insulin may be eligible for a one-time immediate supply of up to three vials or two packs of pens with a prescription.
More Information on Affordable Insulin
Sanofi Insulins Valyou Savings Program offers any of their insulins (Toujeo, Lantus, ADMELOG, and Apidra) for $99/month to those who qualify.
Check out diaTribe’s comprehensive resource on insulin affordability options.
Lilly cuts insulin price by 50% – March, 2019
To stay up to date on the latest medical updates join Coach Beverly on January 21st at 11:30 for her annual State of the Standards Live Webinar.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]A1c or Ambulatory Glucose Profile?
The 2020 ADA Standard Six, reviews Glycemic Targets. Two things caught my eye in this section:
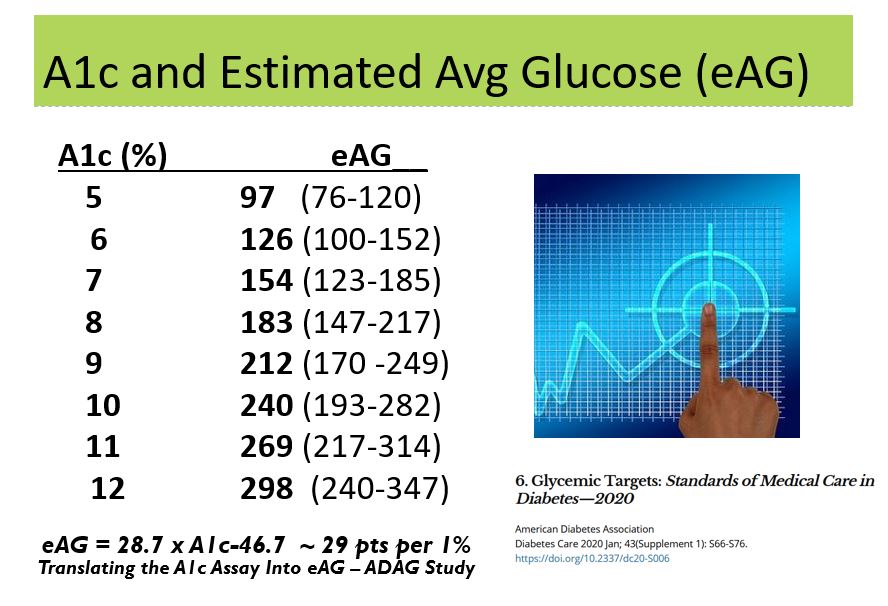
- The updated estimated Average Glucose (eAG) chart not only shows the relationship to A1c and eAG, it also shows the range of glucose for any of the given A1c values. This extra data will certainly be helpful as we discuss the significance and meaning of A1c results with participants.
- Also, for participants utilizing CGM technology, this standard recommends that providers of diabetes care review the Ambulatory Glucose Profile (AGP) report each visit.
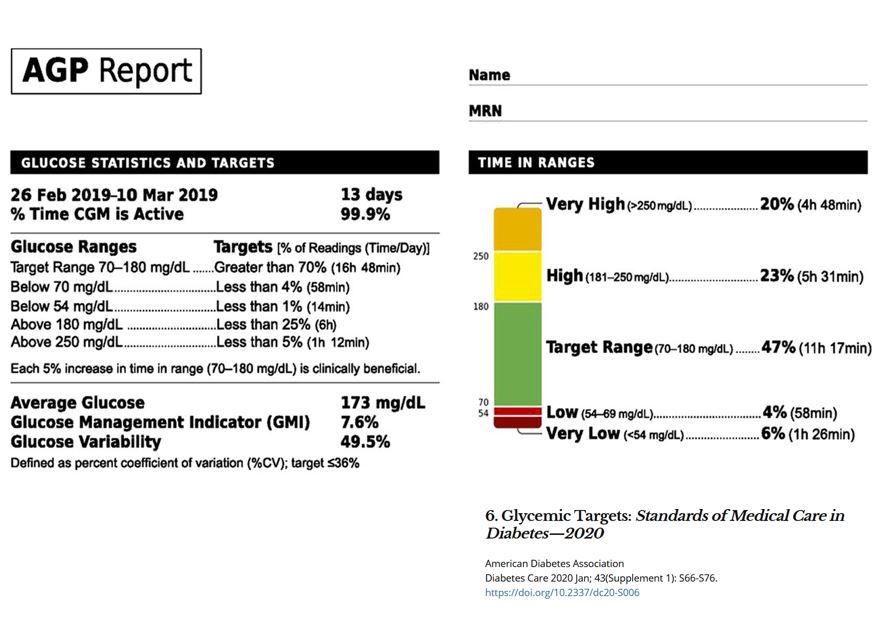
Ambulatory Glucose Profile (AGP) Report benefits: Since the A1C does not provide a measure of glycemic variability or hypoglycemia, the AGP is especially helpful for those prone to bigger glycemic swings.
Glycemic management is best evaluated by the combination of results from SMBG or CGM and A1C. A review of the AGP Report is recommended at each visit for those using CGM technology.
The overall goal is to reach Time in Target Range (70-180) at least 70% percent of the time with minimal hypo and hyperglycemia. In this snapshot ADP Report example below, we see that the A1c is 7.6%, but when we look at the right hand Time in Range side, we quickly note that this individual is struggling with frequent lows and very low glucose levels coupled with hyperglycemia. The time is range is only 47%.
This data is invaluable to help start the process of problem solving to decrease hypo and hyperglycemic events and increase time in target.
An A1c by itself, even if accompanied with frequent blood sugar checks, may not capture this complete picture of 24 hour glucose variability.
See our blog on Time in Range for more info about targets for different groups.
Those are just some of the highlights of the 2020 Standards of Care. Please join Coach Beverly on January 21st at 11:30 for her annual State of the Standards Live Webinar.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Fiasp (faster aspart) approved for Pediatrics
After treat-to-target trial findings showed that mealtime and postprandial treatment with Fiasp resulted in effective glycemic control and safety results similar to slightly slower insulin aspart (Novolog), the FDA has approved Fiasp for use in pediatrics with diabetes.
Fiasp, a rapid-acting human insulin analog, was compared with NovoLog in a randomized trial involving 777 pediatrics (aged 2 to 17 years). There were blind meal injections and open-label postprandial injections. Week 26 of the trial showed both were effective at getting glucose to target.
In children and adolescents with type 1 diabetes, mealtime and postmeal faster aspart with insulin degludec provided effective glycemic control and superior HbA1c with no additional safety risks versus aspart (Novolog).
Read more at PubMed.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Metformin May Prevent Breast & Other Cancers
Multiple studies have examined metformin for its potential tumor and cancer fighting ability. The National Center for Biotechnology Information (NCBI) published such a study in 2016. Researchers found substantial pre-clinical evidence suggesting anti-cancer properties of metformin based on in-vitro and in-vivo analysis. Their analysis suggested metformin could be used as a radiation sensitizer or immunotherapy drug, besides its direct anti-proliferative properties .
In mice with lung cancer, metformin was used and researchers saw a 72% reduction in tumor burden. Tumors are known to exhibit the Warburg effect, but metformin blunts this and consequently downregulates the growth of cancer stem cells.
Several observational studies showed a correlation between metformin use and lessened cancer incidence. The results demonstrated that metformin users have statistically significant reductions in liver, pancreatic, colorectal and breast cancers.
You can read more details on the study and enjoy their infographics and tables of data here.
Coach Beverly reports no conflict of interest for medication postings.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]October Newsletter | New Oral GLP-1: an Easier Pill to Swallow?
October Newsletter Now Available!
Our October Newsletter is brimming with medication updates! From Oral GLP-1 to Semaglutide and pre-filled Glucagon, we’re keeping you up to date AND updating our PocketCards. Did you know you can hire Coach Beverly to come speak in your hometown? Read this month’s Newsletter to find out how to request her time.
October News Topics Include:
- Oral GLP-1: Easier to Stomach than Injectables?
- Canagliflozin Slows Kidney Disease and Protects the Heart
- Diabetes & Breast Cancer
- 25% Off Live Webinars
- Updated DiaBingo
- 7 Strategies to Survive Halloween
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Metformin Decreases All Cause Mortality
A post-analysis of the SAVOR-TIMI 53 trial and a review of 17 different observational studies showed metformin use is associated with decreased all-cause mortality in people with type 2 diabetes and a high risk of cardiovascular events.
In a previous systematic review of 17 observational studies published in the Annals of Internal Medicine in 2017, researchers concluded that metformin use is associated with decreased all-cause mortality in patients with CKD, congestive heart failure, or chronic liver disease with hepatic impairment.
Find out more details about the Harvard Medical School study here.
Want more great medication information? Download our Medication PocketCard for free! We also have this great 8-cards-in-1, laminated, accordion fold PocketCards available with bulk discounts.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Pre-Filled Glucagon Pen Approved by FDA
Xeris Pharmaceuticals new pre-filled glucagon pen, Gvoke, just received FDA’s stamp of approval to treat severely low blood sugar levels in people with diabetes.
The Gvoke glucagon pen is filled with a liquid stable form of glucagon and is approved for use in people with diabetes, age 2 and above. Xeris will have the pre-filled syringe version available in 4-6 weeks and the auto-injector version available in 2020.
The Xeris glucagon pen was created to simplify glucagon injections. This new Gvoke pen does not require any mixing, since it is filled with liquid stable glucagon.
Availability of a glucagon delivery device is critical to treat severe hypoglycemia and prevent serious consequences of untreated severe hypoglycemia such as cardiovascular events, seizure, coma, or even death.
Read more about the Gvoke Glucagon Pen and auto-injector here .
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]








