 “Insulin’s High Cost Leads To Lethal Rationing” – NPR
“Insulin’s High Cost Leads To Lethal Rationing” – NPR
Such a tragic story of a young man’s life cut short because he couldn’t afford his insulin. Sign the ADA Petition to make insulin affordable.
We can make a difference starting today!
Make Insulin Affordable – what you can do today
The Insulin Wars ( How insurance companies farm out their dirty work to doctors and patients) – The New York Times
Nasal glucagon submitted for approval
 According to a statement made recently by Eli Lilly, they have submitted a nasal glucagon treatment to the FDA. The treatment would be for cases of severe hypoglycemia in adults and children with diabetes. This treatment would be the first of it’s kind, a nasal spray, to treat low blood glucose emergencies in those with diabetes.
According to a statement made recently by Eli Lilly, they have submitted a nasal glucagon treatment to the FDA. The treatment would be for cases of severe hypoglycemia in adults and children with diabetes. This treatment would be the first of it’s kind, a nasal spray, to treat low blood glucose emergencies in those with diabetes.
“The submissions put us one step closer to bringing this innovative rescue medicine to the diabetes community and filling an important need in the treatment of severe hypoglycemia,” said Thomas Hardy, Senior Medical Director, Lilly Diabetes, told Endocrine Today.
The way glucagon is currently administered can be a complicated process, requiring the administer to reconstitute the powder and other steps. This can be a confusing process, particularly if you are a caregiver a a child with diabetes. The new nasal glucagon would deliver the medicine in a powder form in an easy, ready to use format. Many people already understand how to use nose spray, making this functional but also realistic in a hypoglycemic emergency.
“This is important and different. You don’t want people to get low [blood sugar], but they do. It’s not a pretend problem, and the fact that the science is clear that people don’t know how to treat it makes it a real problem. With this nasal glucagon kit, anyone could rescue them.”
To learn more about the new nasal glucagon – Lilly submits NDA for nasal glucagon by Helio Endocrine Today
New SGLT-2 Inhibitor – Ertugliflozin
 New SGLT-2 Inhibitor – Ertugliflozin
New SGLT-2 Inhibitor – Ertugliflozin
The Food and Drug Administration (FDA) has approved Ertugliflozin (Steglatro), a new SGLT-2 Inhibitor to treat adults with type 2 diabetes. Also approved are two new combination medications with ertugliflozin. These include Segluromet (ertugliflozin plus metformin) and Steglujan (ertugliflozin plus sitagliptin). See box below for available combination doses (in mgs).
Dosing range: 5 -15 mg daily. Monitor GFR before starting. Don’t start med if GFR is less than 60 and stop if GFR is less than 30.

Considerations: Ertugliflozin has the same warnings as other SGLT-2s: Watch for hypotension, UTI’s, increased urination, genital infections, ketoacidosis.
Benefits: no hypoglyecmia or weight gain.
Lowers A1c 1.0% – 2.0%. Lowers wt 1-3 lbs.
Download our FREE Updated Insulin PocketCard
Medication Update Webinar – Feb 19, 2018 11:30 to 1pm (PST)
Feeling overwhelmed by all the new recently approved diabetes medications? Two bio-similiar insulins are now available and another GLP-1 RA was just approved. Plus, 2 new combo oral meds are now available.
Are you wondering how to apply the new 2018 ADA and AACE Medication Management Guidelines into practice?
We are here to help out!
This webinar will discuss how to integrate these new medications into our practice. We will discuss the benefits and limitations and critical information to share with our patients and providers.
If you want cutting edge information on the latest pharmacology and how to incorporate the new ADA Guidelines into practice, we highly recommend this Meds Update.
To Join Us and earn CEs you have 2 options:
New Copycat Bolus Insulin
New Copycat Bolus Insulin
The Food and Drug Administration (FDA) has approved Admelog (Sanofi-Adventis US), the first copycat version of short-acting insulin lispro (Humalog, Eli Lilly) to treat individuals aged 3 years and older with type 1 diabetes and adults with type 2 diabetes.
Download our FREE Updated Insulin PocketCard
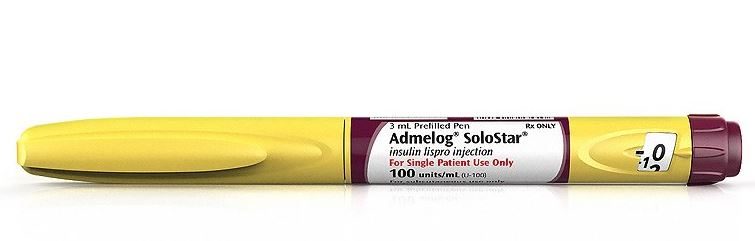 Features:
Features:
Approved for use as an injection, via pump, or intravenously.
It will be available both in vials and as a prefilled pen (Admelog SoloStar).
The FDA approved Admelog through an abbreviated approval pathway under which companies can rely on the FDA’s previous approval of a drug as safe and effective or on previously published literature supporting safety and/or effectiveness. This process is meant to reduce drug development costs in order to reduce the drug’s price on the market.
Medication Update Webinar – Feb 19, 2018 11:30 to 1pm (PST)
Feeling overwhelmed by all the new recently approved diabetes medications? Two bio-similiar insulins are now available and another GLP-1 RA was just approved. Plus, 2 new combo oral meds are now available.
Are you wondering how to apply the new 2018 ADA and AACE Medication Management Guidelines into practice?
We are here to help out!
This webinar will discuss how to integrate these new medications into our practice. We will discuss the benefits and limitations and critical information to share with our patients and providers.
If you want cutting edge information on the latest pharmacology, we highly recommend this Meds Update.
To Join Us and earn CEs you have 2 options:
New GLP-1; Semaglutide (Ozempic)
New GLP-1; Semaglutide (Ozempic)
The US Food and Drug Administration (FDA) has approved semaglutide (Ozempic, Novo Nordisk) as an adjunct to diet and exercise for the treatment of type 2 diabetes in adults.
This once weekly injection of (GLP-1) receptor agonist will be available in 0.5-mg and 1.0-mg doses, via a dedicated prefilled pen device.
Semaglutide is the seventh GLP-1 receptor agonist on the US market and the third dosed once weekly. And the data suggests it might be more effective than some of its competitors.
Download our updated FREE Injectables PocketCard
 Features:
Features:
The company’s eight phase 3a trials involved over 8000 adults with type 2 diabetes, including individuals at high cardiovascular risk and those with renal disease. One of the studies, SUSTAIN-6, was a 2-year FDA-mandated cardiovascular-outcomes trial involving 3297 patients.
- In the five SUSTAIN efficacy trials, semaglutide reduced hemoglobin A1c by 1.5 to 1.8 percentage points.
- Semaglutide was also associated with a 4.5- to 6.4-kg weight loss. The most common side effect was mild to moderate nausea, which diminished over time.
After approval, Novo Nordisk is required to conduct a pediatric trial in adolescents younger than 18 years of age and to add semaglutide to a 15-year medullary thyroid carcinoma registry that includes all of the long-acting GLP-1 products.
Semaglutide will be priced similarly to current weekly GLP-1 receptor agonists and will be offered with a savings card program to reduce copays for eligible commercially insured patients.
Discovery of Insulin – World Diabetes Day
World Diabetes Day Celebrates Dr. Banting and the Discovery of Insulin
 During a hot summer in 1921, Dr.Banting secured space to test out his theory in the University of Toronto. Along with his colleague, Charles Best, and a bare bones lab, they conducted dozens of experiments on dogs, which ultimately led to the discovery of insulin.
During a hot summer in 1921, Dr.Banting secured space to test out his theory in the University of Toronto. Along with his colleague, Charles Best, and a bare bones lab, they conducted dozens of experiments on dogs, which ultimately led to the discovery of insulin.
Dr. Banting and Charles Best began their experiments ligating the pancreases of dogs, thinking this would prevent destruction by the digestive pancreatic juices, and then isolating the extract from the islet cells. They then processed the extract from the islet cells and injected this extract they called “insulin” into diabetic dogs. According to an audio Interview with Dr. Best, by July 1921, they had 75 positive examples of insulin lowering blood glucose levels in dogs.
 In February 1922, doctor Frederick Banting and biochemist John Macleod published their paper on the successful use of a alcohol based pancreatic extract for normalizing blood glucose levels in a human patient.
In February 1922, doctor Frederick Banting and biochemist John Macleod published their paper on the successful use of a alcohol based pancreatic extract for normalizing blood glucose levels in a human patient.
Here are some photos of the first insulin bottles produced by the University of Toronto and Eli Lilly.
Soon, word of their discovery got out and the race was on to produce enough insulin to treat the flood of type 1 patients arriving in Toronto to receive this miracle injection.
But, as with any amazing discovery, there is always more to the story.
One of the biggest barriers to Banting was the simple fact that he was not involved in the field of diabetes research. The idea leading to the discovery of insulin came to him after preparing a lecture on the pancreas and diabetes, a subject he knew little about. He wasn’t a trained researcher and thus securing support for the project was initially difficult.
First Patients to Receive Insulin
The first patient to receive insulin was a ‘welfare’ case at Toronto General Hospital – no clinical trial structure to say the least. People from Canada/US flooded into Toronto to receive treatment. Banting struggled with the lack of accessibility of insulin – volume needed, issues of purification.

The earliest patients were “selected”, some youths from Canada/US, some soldiers with diabetes (probably because of Banting’s service in the First World War) and then later some select private patients. During this time they were working hard to increase the volume and continue to improve the purification process. Insulin was available for testing in US, namely through Dr. Elliot Joslin in the late summer 1922.
Takes a Team
 While Best played a critical and important role, credit must also go to Professor Macleod, from the University of Toronto, who provided the lab space, showed Dr. Banting how to operate on dogs, provided his student Best and suggested they switch from a saline to alcohol to purify the ‘extract’. Dr. Macleod also secured the support of JB Collip, the 4th man on the team and the fist person to purify insulin for human use. Best is also known for pushing Banting to return to the research during a particular dark period of failure.
While Best played a critical and important role, credit must also go to Professor Macleod, from the University of Toronto, who provided the lab space, showed Dr. Banting how to operate on dogs, provided his student Best and suggested they switch from a saline to alcohol to purify the ‘extract’. Dr. Macleod also secured the support of JB Collip, the 4th man on the team and the fist person to purify insulin for human use. Best is also known for pushing Banting to return to the research during a particular dark period of failure.
Dr. Banting – Fun and Interesting Facts
- Sold insulin patent for $1

- Was wounded during the First World War and received the Military Cross
- Youngest Nobel Laureate in Medicine
- First Canadian on the cover of Time Magazine
- Among the last Canadians to receive a knighthood and have the title Sir Frederick Banting
- One of only two “non-Americans” to have a Second World War Liberty Ship named after him (USS Frederick Banting)
- Has as a crater on the Moon named after him (between Apollo 15 & 17 landing sites).
Want to Learn More About the Dr. Banting?
Visit Banting House FaceBook Page
“Metformin Particularly Effective in Those With History of GDM”
Metformin Particularly Effective in Those With History of GDM – check out this article written in Medscape for more information.
These latest findings come from the Diabetes Prevention Program (DPP) and its extension phase.
After 15 years from the start of DPP, women with a history of gestational diabetes taking metformin still had a 41% reduced risk of type 2 diabetes, compared with an 11% reduction in parous women with no history of gestational diabetes.
This contrasts with an overall effect of metformin in reducing the risk of type 2 diabetes by 18% in the study cohort as a whole.
“The overall results reinforce the long-lasting efficacy of metformin in reducing the development of diabetes and support its more widespread use as a prevention measure in those at high risk,” said David M Nathan, MD, director of the Diabetes Center at Massachusetts General Hospital, Boston, the study chair of DPP, who presented these latest results at the conference.
Asked for comment, Shubhada Jagasia, MD, professor of medicine and vice chair of clinical affairs in the department of medicine, Vanderbilt University Medical Center, Nashville, Tennessee, told Medscape Medical News that these new data should help doctors to target metformin treatment to those who will benefit most.
Women with a history of GDM who developed prediabetes that were started on Metformin decreased risk of developing type 2 diabetes by 41% at the 15 year mark. Wow.
Interested in learning more about women and diabetes? Check out our Online University course:
Level 2 – Women and Diabetes 1.0 CEs – $19.00
Women with diabetes are confronted with a variety of issues that require special attention, education and understanding. This course reviews those special needs while focusing on Gestational Diabetes and Pre-Existing Diabetes.





 According to a statement made recently by Eli Lilly, they have submitted a nasal glucagon treatment to the FDA. The treatment would be for cases of severe hypoglycemia in adults and children with diabetes. This treatment would be the first of it’s kind, a nasal spray, to treat low blood glucose emergencies in those with diabetes.
According to a statement made recently by Eli Lilly, they have submitted a nasal glucagon treatment to the FDA. The treatment would be for cases of severe hypoglycemia in adults and children with diabetes. This treatment would be the first of it’s kind, a nasal spray, to treat low blood glucose emergencies in those with diabetes.  New SGLT-2 Inhibitor – Ertugliflozin
New SGLT-2 Inhibitor – Ertugliflozin
 Features:
Features: Features:
Features: During a hot summer in 1921, Dr.Banting secured space to test out his theory in the University of Toronto. Along with his colleague, Charles Best, and a bare bones lab, they conducted dozens of experiments on dogs, which ultimately led to the discovery of insulin.
During a hot summer in 1921, Dr.Banting secured space to test out his theory in the University of Toronto. Along with his colleague, Charles Best, and a bare bones lab, they conducted dozens of experiments on dogs, which ultimately led to the discovery of insulin.  In February 1922, doctor
In February 1922, doctor 
 While Best played a critical and important role, credit must also go to Professor Macleod, from the University of Toronto, who provided the lab space, showed Dr. Banting how to operate on dogs, provided his student Best and suggested they switch from a saline to alcohol to purify the ‘extract’. Dr. Macleod also secured the support of JB Collip, the 4th man on the team and the fist person to purify insulin for human use. Best is also known for pushing Banting to return to the research during a particular dark period of failure.
While Best played a critical and important role, credit must also go to Professor Macleod, from the University of Toronto, who provided the lab space, showed Dr. Banting how to operate on dogs, provided his student Best and suggested they switch from a saline to alcohol to purify the ‘extract’. Dr. Macleod also secured the support of JB Collip, the 4th man on the team and the fist person to purify insulin for human use. Best is also known for pushing Banting to return to the research during a particular dark period of failure.






