
Subscribe
eNewsletter

Ready to get certified?
Free CDCES Coach App
Download
Free Med Pocket Cards

eNewsletter

Free CDCES Coach App
Free Med Pocket Cards
For last week’s practice question, we quizzed participants on recommendations for facilitating positive health behavior change, according to 2026 ADA Standards. 81.75% of respondents chose the best answer. We want to clarify and share this important information, so you can pass it on to people living with diabetes and your colleagues, plus prepare for exam success!
Before we start though, if you don’t want any spoilers and haven’t tried the question yet, you can answer it below: Answer Question
KC has type 2 diabetes, diagnosed 5 years ago. They report low physical activity, frequent sugar-sweetened beverage intake, and high stress related to work. Last A1c was 8.2%. KC reports previous advice to “exercise more and drink less soda,” but reports making minimal changes. They express interest in improving health but feels overwhelmed by where to start.
Which of the following responses best aligns with the 2026 ADA Standards of Care recommendations for facilitating positive health behavior change?
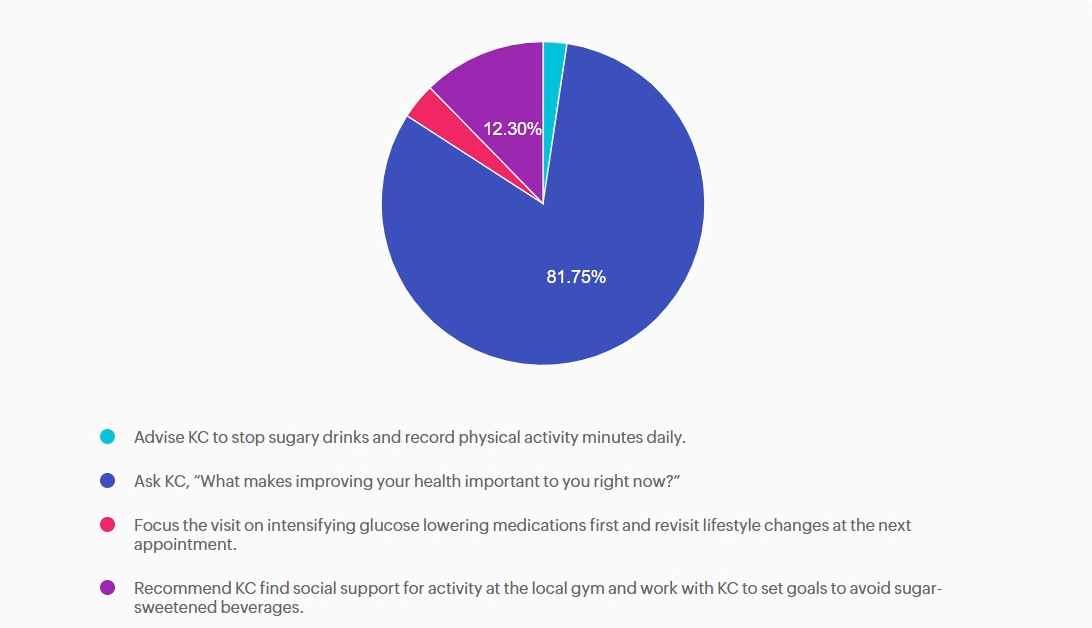
Answer A is incorrect: 2.38% chose this answer, “Advise KC to stop sugary drinks and record physical activity minutes daily.” Although the Standards of Care do recommend avoiding sugar-beverage consumption and increased activity, this answer is prescriptive and not collaborative. Simply telling the patient what to do without assessing motivation or barriers does not align with ADA recommendations for patient-centered behavior change.
Answer B is correct: 81.75% chose this answer, “Ask KC, “What makes improving your health important to you right now?”” This question is an example of a response using the strategy of motivational interviewing (MI). MI is a patient-centered counseling method that explores readiness, addresses ambivalence, helps patients identify barriers to behavior change and encourages confidence while setting goals. The 2026 ADA Standards of Care recommend using MI, along with other strategies, to help individuals with diabetes adopt sustainable lifestyle behaviors, including diet, physical activity, and stress management.
Answer C is incorrect: 3.57% chose this answer, “Focus the visit on intensifying glucose lowering medications first and revisit lifestyle changes at the next appointment.” While medication intensification may be a first step and necessary approach to support KC is their diabetes management, it fails to first address KC’s goals and desires. In addition, the 2026 ADA Standards of Care emphasize integrating behavior change support alongside pharmacologic therapy.
Answer D is incorrect: 12.3% chose this answer, “Recommend KC find social support for activity at the local gym and work with KC to set goals to avoid sugar-sweetened beverages.” While this option does consider strategies of social support and goal-setting it lacks assessment of readiness and barriers and again is a more prescriptive approach vs. collaborative approach.
We hope you appreciate this week’s rationale! Thank you so much for taking the time to answer our Question of the Week and participate in this fun learning activity!

Sale Ends on February 16th, 2026!

A 32-year-old with newly discovered diabetes is brought to the emergency department with polyuria and lethargy. They have been sleeping more than usual.
Initial labs show:
Based on the following labs, which feature most strongly supports a diagnosis of hyperosmolar hyperglycemic state (HHS) rather than diabetes ketoacidosis (DKA)?
For last week’s practice question, we quizzed participants on JR wanting treatment for pancreatic parasites, and what would be the best response. % of respondents chose the best answer. We want to clarify and share this important information, so you can pass it on to people living with diabetes and your colleagues, plus prepare for exam success!
Before we start though, if you don’t want any spoilers and haven’t tried the question yet, you can answer it below: Answer Question

JR is hospitalized with influenza. They have a history of prediabetes but now have persistent glucose readings between 220–260 mg/dL and are started on basal-bolus insulin.
JR is upset and states: “I’ve been reading that pancreatic parasites can cause of diabetes. No one is treating my infection.”
What is the BEST response?
Answer A is correct: % chose this answer, “It sounds like you are worried about a parasite infection. Tell me more about what you’ve read.” Great job. A is the best answer because it uses person-centered, nonjudgmental communication, as recommended by the ADA. It avoids dismissive language and explores misinformation respectfully. It preserves the therapeutic alliance.
Answer B is incorrect: % chose this answer, “Yes. We will be treating that issue soon, but first we need to focus on your insulin doses.” Option B offers a false narrative saying that they are going to treat the parasitic infection then shifts focus to the blood glucose, without recognizing JR’s emotional distress.
Answer C is incorrect: % chose this answer, “I can see how you would be concerned, but here is no such thing as pancreatic parasites.” Option C does initially recognize the emotions but then ends with a dismissive tone, that may make JR feel defensive and unheard.
Answer D is incorrect: % chose this answer, “Sadly, prediabetes always progresses to diabetes when people are acutely ill, and you will probably be discharged on insulin therapy.” Option D completely ignores the emotional distress in addition to making assumptions that may not be true.
We hope you appreciate this week’s rationale! Thank you so much for taking the time to answer our Question of the Week and participate in this fun learning activity!
Get exam-ready with confidence.
Course credits through AMA PRA Category 1 Credits™, ACPE, ANCC, and CDR!
Full accreditation details are available on the registration page

Our CDCES Boot Camp Online Prep Bundle is a comprehensive, high-impact program built specifically for healthcare professionals preparing for the Certified Diabetes Care and Education Specialist (CDCES) exam who want to level up their clinical knowledge and skills.

This evidence-based study bundle is a comprehensive BC-ADM Boot Camp designed for advanced-level healthcare professionals preparing for the Board Certified in Advanced Diabetes Management (BC-ADM) exam and will also provide you with state-of-the-art information to level up your clinical practice.

Join national experts including Dr. Diana Isaacs (Cleveland Clinic), Beverly Thomassian (30+ years of experience), and Christine Craig for high-impact, virtual learning—no travel required.
✔ Learn from National Experts — Anywhere
Get the same expert-level instruction you’d receive in person, delivered live to your home or office.
✔ Interactive & Flexible
Walk away with tools you can apply immediately in clinical practice or while preparing for CDCES or BC-ADM exams. From insulin dosing protocols to behavior change strategies that work in the real world—this content bridges theory and practice.


Making a connection is healing for the person in our care and for us too.
This is why I developed what I call the ABC Worksheet — a simple framework rooted in forging genuine connections rather than focusing on corrections:
This simple ABC approach shifts the tone of care. Instead of asking, “Why aren’t you doing this?” we ask, “I’m curious, what’s the hardest thing about managing your diabetes right now?”

Diabetes is just one aspect of their complete humanity. Diabetes is a common chronic condition that disrupts the usual orchestration of glucose management. No one can give themselves diabetes. And, the individual with diabetes is not to blame for this glucose dysregulation. It’s due to a combination of genetics, early childhood experiences, and social factors that shape health.
I give all of us permission to free ourselves from being responsible for patients’ choices. Our job is to listen and help the individual discover their own internal wisdom. The best way to tap into their self-knowledge is to allow space for reflection and to ask permission before offering our well-intentioned insights.
As I write in my book, “Engaging in active listening is considered a therapeutic intervention on its own. When we focus on listening with curiosity first, something powerful happens. A bridge is built – a connection is made.”
This bridge of active listening provides the perfect segue to dive into diabetes distress and the emotional weightiness of life with diabetes.
Diabetes distress acts like a brake. If we don’t first address fear, shame, or diabetes overwhelm, not even the best diabetes education plan will stick. But when we assess emotional well-being early, we release that brake — and forward movement becomes possible. (Here is a link to Diabetes Distress Screening Tools)
And here is the beautiful part: this approach doesn’t just help the person with diabetes. It sustains us.
“In the end, a person-centered approach doesn’t only support the person with diabetes—it also supports the healthcare professional by fostering authentic connection, shared responsibility, and a greater sense of purpose in your work.”
Investing in connection reduces burnout and increases the likelihood of success. It frees us from being responsible for their choices. We are simply the coach, helping them discover the wisdom they already possess. This partnership can restore our purpose and allow us to be present in this healing journey. Compassion is re-energizing for us and for the person in our care.
So instead of saying, “I know you don’t want to get diabetes complications, so you need to really work on your weight loss goals,” we greet the person and ask them, how are you feeling?
Instead of focusing on their weight at the scale, we ask them, how are you feeling in your body?
Instead of focusing on the weight they have gained, we focus on the behavior changes they have made (meeting with the RDN, eating more veggies, and decreasing their soda intake).
How good does that feel for both the health care provider and the person in their care? It feels great.
Download the ABC’s of Teaching Through Connection Worksheet and begin integrating Awareness, Belonging, and Collaboration into your daily practice.
And if this message resonates with you, I invite you to explore Healing through Connection for Healthcare Professionals. Coach Beverly wrote this book to help you care for others while finding yourself in the process.
👉 Download the Worksheet
👉 Order the Book Here
Let’s move from judgment to partnership.
Let’s lead with connection and curiosity.
Coach Bev 💜

Join national experts including Dr. Diana Isaacs (Cleveland Clinic), Beverly Thomassian (30+ years of experience), and Christine Craig for high-impact, virtual learning—no travel required.
✔ Learn from National Experts — Anywhere
Get the same expert-level instruction you’d receive in person, delivered live to your home or office.
✔ Interactive & Flexible
Walk away with tools you can apply immediately in clinical practice or while preparing for CDCES or BC-ADM exams. From insulin dosing protocols to behavior change strategies that work in the real world—this content bridges theory & practice.

Get exam-ready with confidence.
Course credits through AMA PRA Category 1 Credits™, ACPE, ANCC, and CDR!
Full accreditation details are available on the registration page

Our CDCES Boot Camp Online Prep Bundle is a comprehensive, high-impact program built specifically for healthcare professionals preparing for the Certified Diabetes Care and Education Specialist (CDCES) exam who want to level up their clinical knowledge and skills.

This evidence-based study bundle is a comprehensive BC-ADM Boot Camp designed for advanced-level healthcare professionals preparing for the Board Certified in Advanced Diabetes Management (BC-ADM) exam and will also provide you with state-of-the-art information to level up your clinical practice.


For last week’s practice question, we quizzed participants on J.C.’s family history and lab work, and what it reveals. 80.92% of respondents chose the best answer. We want to clarify and share this important information, so you can pass it on to people living with diabetes and your colleagues, plus prepare for exam success!
Before we start though, if you don’t want any spoilers and haven’t tried the question yet, you can answer it below: Answer Question

J.C. is a ten-year-old female with a family history of type 1 diabetes. Her 7-year-old brother was diagnosed with type 1 diabetes two years ago. J.C. has no complaints and reports feeling well. She enjoys playing sports, including basketball and soccer. Her current BMI is 22.1 (93rd percentile for age). She denies any polydipsia, polyuria, or polyphagia. Her lab work demonstrates a fasting blood sugar of 71 mg/dL, an A1c of 5.0%, normal kidney function, and normal electrolytes. Her diabetes autoantibody panel shows positive glutamic acid decarboxylase (GAD) and islet antigen 2 (IA-2) antibodies, negative zinc transporter 8 (ZnT8) antibodies, and negative insulin antibodies.
What does her lab work reveal?
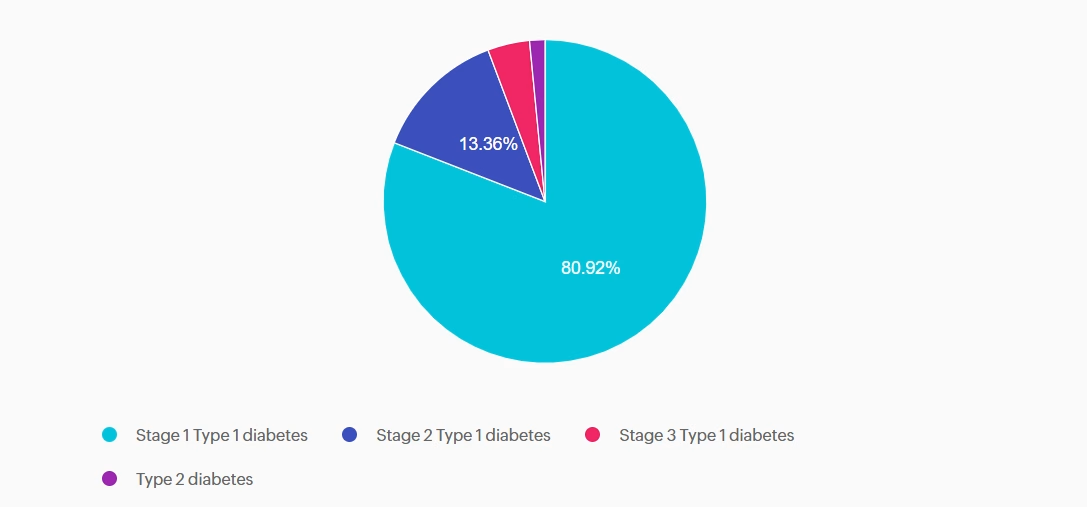
Answer A is correct: 80.92% chose this answer, “Stage 1 Type 1 diabetes.” J.C. has stage 1 type 1 diabetes. She has two positive autoantibodies and normoglycemia.
Answer B is incorrect: 13.36% chose this answer, “Stage 2 Type 1 diabetes.” J.C. still has normoglycemia. Stage 2 type 1 diabetes is characterized by positive autoantibodies and dysglycemia (Impaired fasting glucose, Impaired glucose tolerance, or elevated A1c over 5.7% or 10% increase in A1C).
Answer C is incorrect: 4.2% chose this answer, “Stage 3 Type 1 diabetes.” J.C. does not have lab work confirming diabetes by the standard diagnostic criteria, and she is asymptomatic. Stage 3 type 1 diabetes is characterized by overt hyperglycemia and symptoms of diabetes with autoimmunity present.
Answer D is incorrect: 1.53% chose this answer, “Type 2 diabetes.” J.C. does not have type 2 diabetes. She does have a BMI in the overweight category, but she does not have hyperglycemia. She also has positive autoantibodies associated with type 1 diabetes. Type 2 diabetes is not immune-mediated.
We hope you appreciate this week’s rationale! Thank you so much for taking the time to answer our Question of the Week and participate in this fun learning activity!

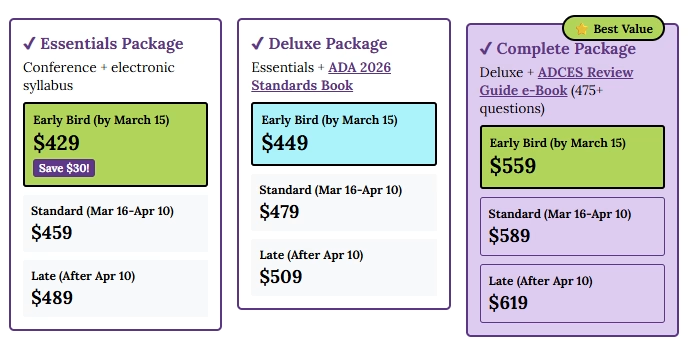
Welcome to our selection of comprehensive CDCES Boot Camp Online Prep Bundles that are specifically designed for healthcare professionals who are studying for the Certified Diabetes Care and Education Specialist (CDCES) exam.
Course credits through AMA PRA Category 1 Credits™, ACPE, ANCC, and CDR!
We offer a selection of prep bundles to meet everyone’s needs! See the descriptions below to review what is included in each option.
CDCES Boot Camp | Basic Exam Prep Bundle: This option is perfect for someone who wants just the Online Courses and materials all in one place, our Online University. This bundle includes Levels 1, 2, and 3 & Toolkits which equates to over 30 courses, 50 CEs/CPEUs, and 400+ online practice questions.
CDCES Boot Camp | e-Deluxe Exam Prep Bundle: This bundle has all of the courses from the Basic Bundle, along with the ADCES Certification Review Guide Practice Questions e-book with 400+ practice questions.

Digital literacy is a key social determinant of health, yet up to one-third of Americans struggle with basic digital literacy skills.¹ In diabetes care, this means many struggle with using, understanding, and applying digital information to improve glycemic control.² As diabetes healthcare providers, how do we know if our clients have low digital literacy? Here are five signs of low digital literacy to watch for in your practice.
Diabetes healthcare professionals are vital in supporting clients to improve their digital literacy.
✅Acknowledge their fears and concerns. Ask open-ended questions to understand their concerns. During your conversations, address any myths or misconceptions they may have about diabetes technology.
✅ Offer support and assistance with improving their digital literacy skills. Whether it is one-on-one education or in group classes, offer device education sessions tailored to those with low digital literacy.
✅ Increase the frequency of phone calls or in-person check-ins to monitor progress with the diabetes device. Support the client at every step of their learning journey and help them stay accountable for their personal diabetes goals.
References:

Gain fresh insights, practical tools, and a deeper understanding of the latest in person-centered diabetes care. Our expert team brings the ADA Standards of Care to life—covering medications, behavior change, technology, and more!
If you’re preparing for the CDCES or BC-ADM exam, this conference, paired with a handful of free bonus courses, serves as the ideal study companion! Plus, this content counts toward the ADA Standards requirements for CDCES Renewal.
With interactive co-teaching, we keep sessions engaging, relevant, and fun.
Let’s learn and grow together!
Course credits through AMA PRA Category 1 Credits™, ACPE, ANCC, and CDR!
Program Objectives:
Upon completion of this activity, participants should be able to:

KC has type 2 diabetes, diagnosed 5 years ago. They report low physical activity, frequent sugar-sweetened beverage intake, and high stress related to work. Last A1c was 8.2%. KC reports previous advice to “exercise more and drink less soda,” but reports making minimal changes. They express interest in improving health but feels overwhelmed by where to start.
Which of the following responses best aligns with the 2026 ADA Standards of Care recommendations for facilitating positive health behavior change?
Course credits through AMA PRA Category 1 Credits™, ACPE, ANCC, and CDR!
Get exam-ready with confidence.
Our CDCES Boot Camp Online Prep Bundle is a comprehensive, high-impact program built specifically for healthcare professionals preparing for the Certified Diabetes Care and Education Specialist (CDCES) exam who want to level-up their clinical knowledge and skills.
✔ Learn at your pace with expert-led, exam-focused content
✔ Everything you need—organized, practical, and in one place
✔ Perfect for self-directed learners who want complete, person-centered content for clinical practice and exam prep.
✔ Build knowledge, sharpen test-taking skills, and prepare with confidence—on your schedule.
Focused. Flexible. Proven.
Basic & e-Deluxe CDCES Boot Camp Bundle Includes:
CDCES Boot Camp | 50+ CEs
Starting: $449 | Starting Sale Price: $381.65
BC-ADM Boot Camp | 50+ CEs
Starting: $459 | Starting Sale Price: $390.15
Dual-Cert Boot Camp | 60+ CEs
Starting: $519 | Starting Sale Price: $441.15
Virtual Training | 30+ CEs
Starting: $429 | Starting Sale Price: $364.65
Sale Ends on February 16th, 2026!

For last week’s practice question, we quizzed participants on what needs to be included in the initial screening for PAD, according to ADA Standards. 56.08% of respondents chose the best answer. We want to clarify and share this important information, so you can pass it on to people living with diabetes and your colleagues, plus prepare for exam success!
Before we start though, if you don’t want any spoilers and haven’t tried the question yet, you can answer it below: Answer Question

Peripheral Artery Disease (PAD) is significantly underdiagnosed. While PAD affects around 8.5 million Americans and prevalence rises with age (up to 20% over 60), only 10-20% are clinically diagnosed, highlighting a major gap in awareness and screening.
According to the ADA Standards, what needs to be included in the initial screening for PAD?
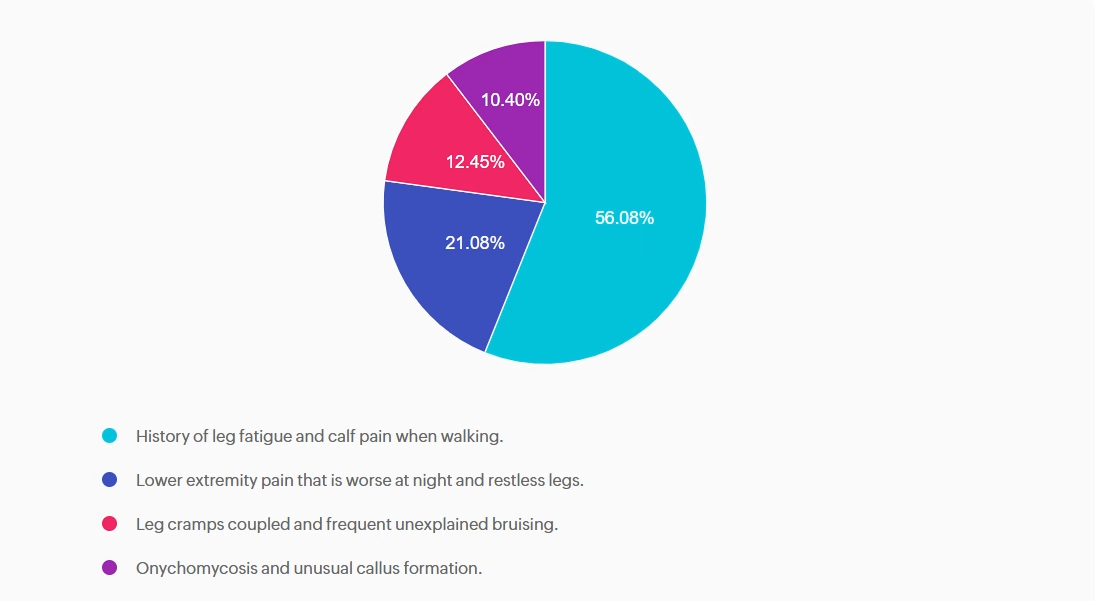
Answer A is correct: 56.08% chose this answer, “History of leg fatigue and calf pain when walking.” YES, great job. According to the ADA, if a person presents with leg fatigue and intermittent claudication, a more detailed screening for peripheral arterial disease (PAD) and poor arterial circulation is warranted.
Answer B is incorrect: 21.08% chose this answer, “Lower extremity pain that is worse at night and restless legs.” People experiencing neuropathy will complain of leg pain and burning that is worse when resting. People with PAD complain of leg and buttock pain when walking due to diminished circulation and poor blood flow to muscles that is relieved with rest.
Answer C is incorrect: 12.45% chose this answer, “Leg cramps coupled and frequent unexplained bruising.” Only part of this answer is correct. Although leg cramps or calf pain can occur with PAD, there is not direct association with frequent unexplained bruising.
Answer D is incorrect: 10.4% chose this answer, “Onychomycosis and unusual callus formation.” People with diabetes do have an increased risk of toenail infections and onychomycosis, but this is not associated with the manifestations of PAD.
We hope you appreciate this week’s rationale! Thank you so much for taking the time to answer our Question of the Week and participate in this fun learning activity!