COVID-19 and Diabetes: CGMs in Hospital Improve Care | Tech Thursday
What does it feel like to be ill with COVID-19 as a person with diabetes? Much of the novel virus COVID-19 is still a mystery that we learn more about every day.

Through the stories of those who have contracted COVID-19, we know that the impacts vary. Symptoms can appear mild with a slight fever and some coughing, like Andrew O’Dwyer from the UK experienced.
While for others symptoms can be more severe, like how a secondary-school teacher living with diabetes had. She had a much longer list of symptoms including difficulty breathing, dizziness, dehydration, and vomiting.
Though even with her more severe symptoms, she struggled with whether she should go to the hospital or not.
“I’m unsure whether to go to the hospital. I feel as though I’ll be wasting valuable resources and I may be an infection risk to vulnerable patients.
As many medical systems are overwhelmed and resources continue to be limited, it’s important to think of ways to reduce the risk of transmission so individuals can get the care they need. Because despite this wide range of impact, COVID-19 continues to emerge with very severe complications for people with or without diabetes.
Reduce the Risk: Glucose control is key!
For people with diabetes who are treating COVID-19, glucose control is key! Keeping BG levels as close to the target range as possible can help reduce the inflammatory response, caused by hyperglycemia. Following the basic guidelines of sick day management will assist the type 1 person who might be diagnosed with COVID-19.
To minimize the risk of transmission, hospitals are starting to use CGM’s for glucose checks in ICUs and in COVID-19 units.
“We knew we needed to get creative” states Carol Levy, MD, Clinical Director of the Mount Sinai Diabetes Center, while discussing “a new initiative to utilize CGM for critically ill patients with COVID-19 to reduce patient-provider contact, conserve PPE, and reduce risks for virus transmission.”
For more information, please see our Emergency Preparedness Blog Post.
There is an incredible amount of information regarding COVID-19 and diabetes. These articles show a glimmer of hope for all people with diabetes that might develop COVID-19.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
To read more Mount Sinai’s efforts click here. Click here for For Arthur’s full story, or for the secondary school’s story click here.
*From ADA Treatment & Care Fact Sheet, “People with diabetes are not more likely to get COVID-19 than the general population. The problem people with diabetes face is primarily a problem of worse outcomes, not a greater chance of contracting the virus. In China, where most cases have occurred so far, people with diabetes had much higher rates of serious complications than people without diabetes.” To help friends and family keep safe, here is an excellent handout on Keeping Safe and Home and in the Workplace by the World Health Organization.
Technology Thursday | Medtronic Announces Latest Diabetes System
Medtronic announces the launch of their Next-Gen Bluetooth automated insulin delivery (AID) and CGM systems!
At last year’s Advanced Technologies and Treatment for Diabetes (ATTD) Conference in Berlin, Germany, Medtronic announced that they will be launching their Next-Gen of AID and CGM systems while sharing the results of a recent study they conducted on children who use their 670G AID system.
The study conducted evaluated the efficiency of the 670G AID (automated insulin delivery) system in children from 2-6 years old. The results were positive. After 3 months, the participants:
- Reduced their A1c’s by 0.5% (from a baseline of 8.0%)
- Increased their “time in range” by 2 hours per day
- Had no change in time spent in less than 70 mg/dl
- No experiences of severe hypoglycemia or DKA
This is exciting news for this age group of 2-6-year-old children and their caretakers as it provides a safe and convenient way of managing diabetes in young kids.
Next-Gen AIDs & CGMs
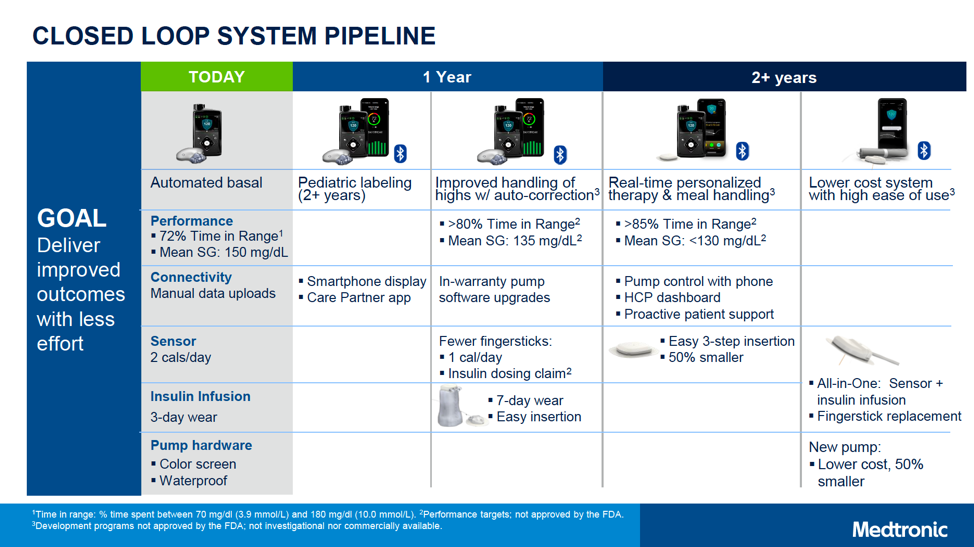
In addition to this great news for younger kids and their families, Medtronic shared several products that they plan to launch over the next several years, phased into “1 year” and “2+ year” timelines.
These products hope to offer a wide range of new features, including:
- An 85% or greater time-in-range and an average glucose of less than 130 mg/dl from their personalized closed loops
- 50% smaller CGM sensors & pumps at a lower cost
- Smartphone app
- All-in-one CGM sensor and insulin infusion set
- Infusion sets with up to seven days of wear
This is such positive information in the on-going advances of CGM and automated insulin delivery systems for children and adults with type 1 diabetes.
Click here for in-depth details of these advances.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Insulin Drone Delivery? | Tech Thursday

The world’s first drone delivery of insulin may be a useful model in response to the COVID-19 pandemic.
For people in rural, remote and isolated urban areas, using a drone to deliver much-needed insulin supplies is an option under consideration. A test flight in Ireland used a drone to deliver insulin as a response to people who were stranded without insulin during Hurricane Ophelia and the post-winter storm Emma when people were snowed in.
“Drone delivery has endless possibilities and can help us connect with our patient communities even in the most remote areas during sentinel events such as hurricanes, earthquakes, and pandemics, which have unfortunately become more common,” Spyridoula Maraka, MD, MS told Healio/Endo.
Though drone delivery has endless possibilities, there are also significant regulatory challenges with “aviation, medication dispensing, pharmaceutical dispensing, and cold chain protocols,” that had to be accounted for during this test run. Markara explains that through each phase of the mission, they had to have backup procedures in place.
Even with the obstacles for drone delivery of insulin or life-saving medications, this is an innovative and exciting prospect for people living in remote or isolated areas.
During the current pandemic, endocrinologists and care providers encourage people that use insulin to have enough stores at home for prolonged “Stay At Home” orders. As stated in our recent Question of the Week people with diabetes are not necessarily at increased risk for contracting COVID-19, but are at risk for experiencing worse outcomes and series complications from the virus (click here to review ADA’s Treatment & Care Factsheet). People living with diabetes may also experience a compromised immune system if blood glucose levels are running above target for a prolonged amount of time.
For these reasons, it is of utmost importance to have the necessary supplies and insulin one needs for optimal glucose levels during periods of crisis, like a pandemic.
To read more, click here and here for more valuable information regarding staying prepared in the pandemic with your insulin and diabetes supplies.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]isCGMs May Reduce Hospital Visits | Tech Thursday
A Belgian study shows that intermittently scanned CGMs (isCGM) can reduce episodes of hypoglycemia and diabetes-related hospital visits in people with type 1 diabetes which can improve or stabilize their quality of life.
In this study conducted by Pieter Gillard, MD, Ph.D., the assistant head of the clinic in the department of endocrinology at University Hospitals Leuven and associate professor at the University of Leuven and colleagues, they reviewed episodes of hypoglycemia, DKA and A1c levels and quality of life measures in a study of 1,913 adult patients with type 1 diabetes.
There were decreases noted in hypoglycemia, DKA, diabetes-related hospital visits, and work sick days. The “quality of life” measures were noted as “overall stable;” A1c levels did not appear to reflect any changes in study participants.
Users of isCGM rated the system as much more convenient than fingerstick glucose testing. Although A1c levels did not change, people using isCGM had greater satisfaction in managing their diabetes.
“Patients prefer to use isCGM compared to fingersticks” – Anne Peters, MD, Director, USC Clinical Diabetes Program
Using an “isCGM” can be helpful for clinicians, CDCES, nurses, and patients in reviewing BG levels stored within the isCGM device.
Click here for more information.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]
Emergency Preparedness & Diabetes | CDC, ADA, & DDRC Advisement

Taking care of ourselves, our families and our patients are our first priorities at this time.
It is very quiet in most neighborhoods this morning; no commuter cars on the streets, no school bus noises, no sounds of children playing. This desolate environment reminds us that there is a lot of unknown for all of us.
It is so important right now to make sure we are all prepared for social distancing and flattening the curve of the spread of this pathogen.
We want to offer a few resources for those living with diabetes to prepare for what’s ahead and how to manage stress during this time. Endocrinologists are urging people that use insulin to plan ahead and have extra supplies on hand.
- DDRC Emergency Preparedness Kit
- ADA Treatment & Care Fact Sheet
- CDC Guidelines for Protecting Yourself and Others from the Coronavirus
- CDC Steps to Take if You’re Sick
- CDC Tips for Managing Stress
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]
Tech Thursday | Improving Access to Diabetes Health Tech

A new series of recommendations from JDRF/UK has set out to help ensure people with type 1 diabetes can improve access to wearable technology to manage their diabetes.
JDRF Pathway to Choice program released a report capturing the barriers, motivations, and opportunities of people with type 1 diabetes regarding medical technology.
This program in the UK aims to build awareness and access to insulin pumps, CGM’s and Flash glucose meters for persons with type 1 diabetes.
The report has three recommendations;
- More patient time spent with specialist healthcare educators.
- Mandatory training covering type 1 diabetes technology for doctors and nurses.
- Clinical commissioning groups to reach out to lower socio-economic groups that might be overlooked.
Karen Addington, JDRF UK’s Chief Executive states, “JDRF believes everyone who wants or would benefit from type 1 diabetes technology should gain access to it”.
As diabetes educators, nurses, doctors, dietitians, and care-givers, we support this effort in the UK for expanding access to technology that can assist people with type 1 diabetes for tighter, healthier glucose control.
Looking forward to the time that this access might be available for all persons with type 1 diabetes!
Read more by clicking here.
Written by Catherine Cullinane RN, CDCES, our resident Technology Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Tech Thursday | New t:slim X2 Insulin Pumps with Control-IQ Technology
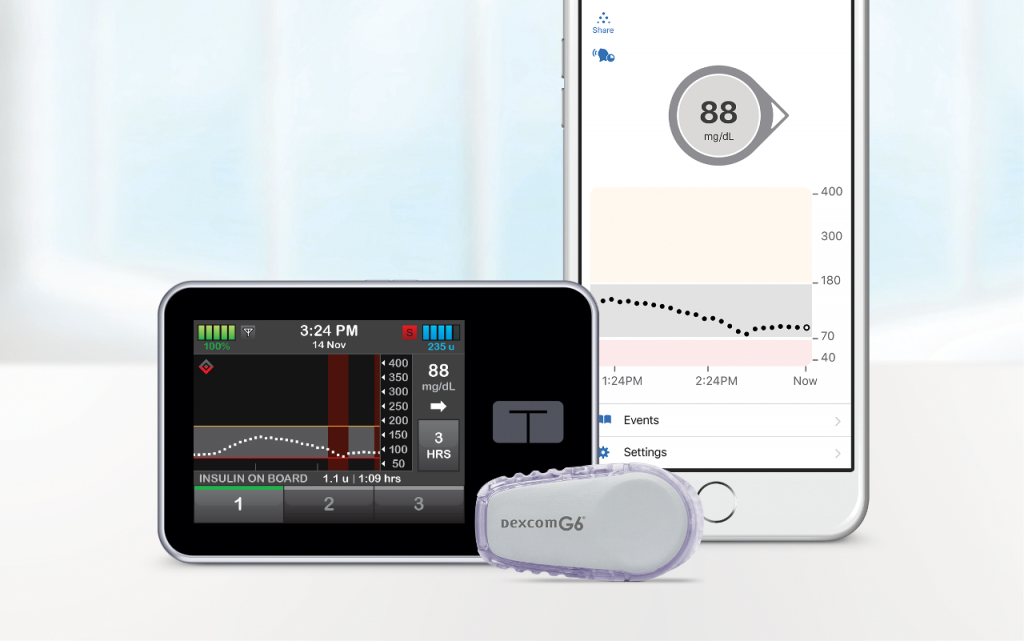
Tandem Diabetes Care announced the commercial USA launch of the t:slim X2 Insulin Pump with Control-IQ Technology.
The Tandem t:slim X2 with Control-IQ is a hybrid closed-loop insulin delivery system.
The Tandem t:slim X2 with Control-IQ is first and only system cleared to deliver automatic correction boluses in addition to adjusting insulin to prevent high and low blood glucose levels. The system integrates with the Dexcom G6 CGM which requires no fingersticks for calibration or diabetes treatment decisions. The goal is to increase time in range (70-180 mg/dL) for users.
Tandem President & CEO John Sheridan stated study participants and investigators have described Control-IQ Technology as “life-changing,” “easy to use,” and “a new standard of care in insulin therapy management.”
Control-IQ Technology system:
- Adjusts insulin delivery to help prevent high and low blood glucose levels
- Automatically delivers a correction bolus
- Features Exercise and Sleep activities
- Requires zero fingersticks with using the Dexcom G6 CGM
Other variables used in this hybrid closed system are:
- insulin on board
- predicting rise and fall of blood glucose levels
- suspending basal rate if a drop in glucose level is predicted
- increasing basal rate if elevated blood glucose levels are predicted
Individual emails are being sent out to in-warranty t:slim X2 users for possible upgrades.
Technology is moving forward with improved automation and usability which is good news for people living with diabetes.
Read more here.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]Technology Thursday | Keeping an “Eye” on Diabetic Retinopathy

Diabetes technology moves forward with the creation of an LED contact lens that can diagnose diabetes and assists with the treatment of diabetic retinopathy. A research team from Pohung University of Science and Technology in South Korea has developed a smart light-emitting diode (LED) contact lens for diagnosis of diabetes and treatment of diabetic retinopathy.
The contact lens will not be able to do a real-time blood glucose check but the lens could offer treatment for retinopathy. There is a possible commercialization in the future in collaboration with PIH Biomed and Stanford University.
On the same note: Apple’s science department has also been working on a contact lens to check a blood glucose level in real-time. It is considered to be one of their “secret” research projects from 2017.
As is often the case, a fingerstick BG would be the standard for the most accurate glucose levels for now. The potential for future developments is exciting!
We will keep an “eye” out for future developments in this exciting field of diabetes research.
Read more at Verdict Medical Devices & CNBC.
Written by Catherine Cullinane RN, CDCES, our resident Tech Thursday Content Writer
Welcome Catherine Cullinane RN, CDCES, our new Technology Thursday Content Writer!

Catherine has been a nurse for 30 plus years, and a Diabetes Educator for 20 years. Her passion is helping people empower themselves with behavioral change for optimal health. Type 1 diabetes management ( insulin pumps, CGMs, and new diabetes technology) is one of her major interests and focus.
Catherine has been a Program Coordinator for the American Diabetes Association’s Education Recognition Program in both Wyoming, and San Francisco, California. She has worked in out-patient clinics, collaborated with hospital in-patient diabetes management, and is a pump and CGM trainer.
She has traveled the world with her own insulin pump, and more recently a CGM. The latest and greatest in type 1 diabetes management continues to amaze her. Catherine loves to travel, rock climb, hike up mountains, read, cook and eat healthy foods.
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]