Download Free Diabetes Cheat Sheets!
Meds PocketCard Refresh for 2026!
New CDCES Coach App – Download Yours Today!
Simplifying Insulin Delivery with CeQur Simplicity
Beta Bionics iLet Bionic Pancreas: Celebrating Freedom from Carb Counting
New Tubeless Insulin Delivery System Approved for Toddlers
The FDA just authorized the Insulet Omnipod 5 tubeless insulin delivery system for pediatrics with type 1 diabetes starting as young as age 2. This remarkable moment marks the availability of the world’s first tubeless, wearable insulin delivery system for toddlers.
The Omnipod 5 received FDA approval earlier this year for integration with the Dexcom G6 continuous glucose monitor for adults and kids with type 1, but Insulet also petitioned the FDA to approve this technology for toddlers.
First Tubeless Hybrid Closed Loop System
This first tubeless hybrid closed loop system to receive FDA authorization includes the Omnipod 5, Dexcom G6 CGM and a phone app or controller to manage insulin delivery and monitor glucose levels. The Omnipod holds three days worth of insulin in a pod delivery device without the need for tubing, which can be helpful for active toddlers. No calibration or fingersticks are required.
As a hybrid loop system, the Omnipod and Dexcom are continuously data sharing, so most of the insulin delivery is automated. However, users can make needed adjustments, deliver bolus insulin and easily share data with the smartphone app or Ominpod 5 Controller.
Promising Outcome Data
Based on a study presented at the annual ADA Scientific Session, children with type 1 diabetes experienced a drop in A1C levels from a baseline of 7.4% to 6.9% and they remained at this improved A1C level for one year into the study. Time in range also increased from 57% at baseline to 68% after using the Omnipod 5 for three months. In addition, there were no reported cases of DKA or severe hypoglycemia. For more information on the latest in insulin pumps and CGMs, please join our Virtual Conference with technology expert Diana Issacs. In addition to explaining the technology, she provides an awesome “show and tell”.
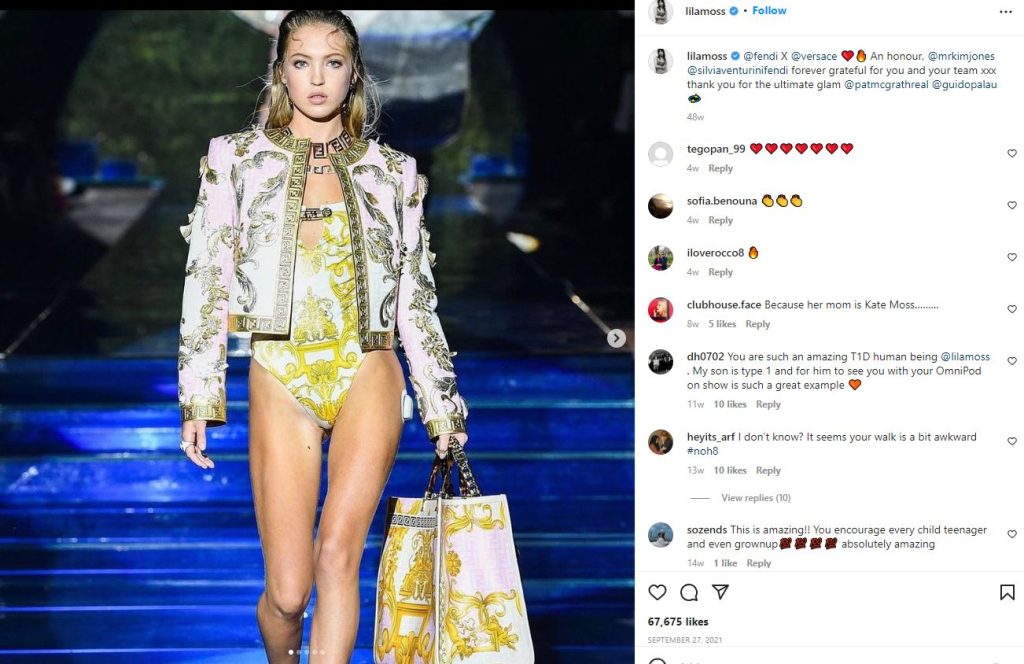
Lila Grace Moss wears Omnipod on Runway
On a related note, I would like to give recognition to the courageous Lila Grace Moss, who lives with type 1 diabetes. On a recent runway show, she did not shy away from wearing her Omnipod as she modeled the latest fashion styles with camera’s clicking and hundreds of audience members.
Social media took note and many instagram followers thanked Lila for her bold and brave statement.
Thanks Lila!
Want to learn more about Technology and Diabetes Care? Join us for our
Virtual DiabetesEd Training Conference
30+ CEs
Airs October 12-14th, 2022
Join us LIVE for this Virtual Training Conference and enjoy a sense of community!
Whether you are new to diabetes or a seasoned expert, you’ll benefit from this virtual conference with the latest research plus critical content that you can immediately apply to your clinical practice.
Download Course Flyer | Download Schedule
If you are seeking a state-of-the-art review of current diabetes care, this course is for you. Our team has been fine-tuning this course for over fifteen years, and we know what you need. This program can also be a great addition to your CDCES or BC-ADM exam study plan.
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
Two Registration Options
Don’t worry if you can’t make it live. Your registration guarantees access to the recorded version in the Online University.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
A CGM that only needs Changing Twice a Year?
An implantable Continuous Glucose Monitor about the size of a matchstick, that only needs to be changed every 180 days, received approved by the FDA this February.
Eversense E3 will be available later this year, for people 18 and over. This implanted sensor determines interstitial glucose readings for 180 days. Sensor data is captured by a small transmitter patch that adheres to the skin, near the implanted device. The transmitter is rechargeable and sends glucose readings data via bluetooth connection to a mobile device. Users can download the mobile app on both iOS and Android devices to monitor glucose trends every 5 minutes and set custom alarms for highs and lows.
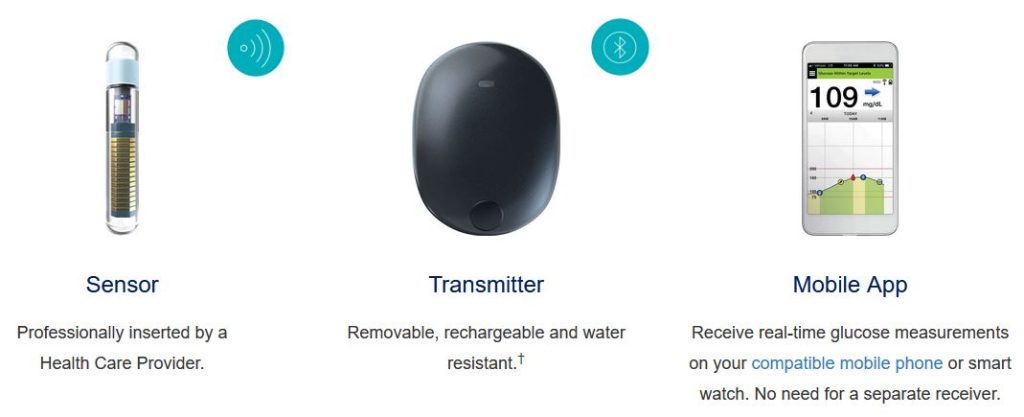
The transmitter patch is changed daily and can be set to vibrate as an alarm to alert the user of hypo and hyperglycemia.
To assure accuracy, sensor calibration is required twice per day for the first 21 days of wear. After day 21, once a day calibration a day is required.
In the PROMISE study, researchers analyzed the safety and accuracy of the Eversense E3 in 181 adults age 18 and up. Results showed that the Eversense E3 was very accurate, with a MARD of 8.5%. The accuracy of the Eversense E3 was also better than any currently available CGM on the market – though it does require daily meter calibrations.
According to Senseonics, the medical technology company that created the implantable device, the out-of-pocket cost will be the same as their original 90-day sensor. Most insurance companies should cover the cost of the device and for insertion and removal.
Senseonics has partnered with Ascencia Diabetes Care (the maker of the Contour blood glucose meters). Ascencia will handle the marketing and distribution of the Eversense products. Plus, Ascencia will offer a Patient Assistance Program that can save users up to $1,200 a year. To learn more, you can contact Ascencia here.
To read more about the Eversense E3, click here.
Want to learn more about this topic and more?
Join us and benefit from Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES, an expert who will be delving into medications and side effects.
Virtual DiabetesEd Specialist Conference
30+ CEs | April 13-15, 2022

Whether you are new to diabetes or a seasoned expert, you’ll benefit from this virtual conference with the latest research plus critical content that you can immediately apply to your clinical practice.
Download Course Schedule | Download Course Flyer
If you are seeking a state-of-the-art review of current diabetes care, this course is for you. Our team has been fine-tuning this course for over fifteen years, and we know what you need. This program can also be a great addition to your CDCES or BC-ADM exam study plan.
Join us LIVE for this Virtual Course and enjoy a sense of community!
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
Two Registration Options
Virtual DiabetesEd Specialist Conference Deluxe | 30+ CEs
Deluxe Option for $499: Virtual Program includes:
- Q & A Session with the instructor after each webinar.
- LIVE Presentations by our team of experts.
- State of the art review of current diabetes care and technology.
- Resources for each session.
- Access to free podcasts and video recordings within a week of each live session for one year.
Deluxe Version includes Syllabus, Standards and Swag*:
- Diabetes Educator Course 2022 Syllabus Hard Copy – over 100 pages -This spiral-bound workbook contains the printed version of all of the instructor’s slides.
- ADA 2022 Standards of Care Book -The ADA Standards of Medical Care in Diabetes is a key resource for healthcare professionals involved in diabetes care, education, and support.
- DiabetesEd Services highlighters, Medication PocketCard, Tote Bag and Pen
Virtual DiabetesEd Specialist Conference Basic | 30+ CEs
Deluxe Option for $499: Virtual Program includes:
- Q & A Session with the instructor after each webinar.
- LIVE Presentations by our team of experts.
- State of the art review of current diabetes care and technology.
- Resources for each session.
- Access to free podcasts and video recordings within a week of each live session for one year.
Don’t worry if you can’t make it live. Your registration guarantees access to the recorded version in the Online University.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
The OmniPod and DexCom Finally Hook Up (and no tubing)
The first tubeless Automated Insulin Delivery (AID) System, was just approved by the Food and Drug Administration (FDA).
The Omnipod 5 hybrid closed-loop system (Insulet Corporation) links with Dexcom’s G6 Continuous Glucose Monitor and is now FDA approved for people with type 1 diabetes age six and older.
This anxiously awaited approval now means that there are 3 semi-automated closed-loop insulin delivery systems in the United States.
A unique feature of the Omnipod 5 system, is that it includes the first approved tubing free insulin delivery device that is paired with the Dexcom G6 continuous glucose monitor (CGM). The Omnipod holds up to 3 days of insulin and is worn directly on the body.
Omnipod 5 Users can monitor glucose trends and deliver insulin using a smartphone app or a separate controller device. Plus, this SmartAdjust technology automatically adjusts insulin to keep glucose on target and minimize hypo and hyperglycemic events.
Included in the app is a SmartBolus calculator that receives Dexcom CGM values every 5 minutes and automatically adjusts insulin based on predicted values for 60 minutes into the future and the individual’s customized glucose targets.
Insulet said it will begin selling the prescription Omnipod 5 device through U.S. pharmacies, starting with an initially limited release before expanding its commercial reach. The company is also offering users of its current Omnipod DASH system—a more manual tubeless pump—an upgrade to the Omnipod 5 at no additional cost once insurance coverage becomes available.
Read more here Omniopod approved by FDA.
Want to learn more about this topic?
Join our Virtual DiabetesEd Specialist Conference
30+ CEs | April 13-15, 2022

Whether you are new to diabetes or a seasoned expert, you’ll benefit from this virtual conference with the latest research plus critical content that you can immediately apply to your clinical practice.
If you are seeking a state-of-the-art review of current diabetes care, this course is for you. Our team has been fine-tuning this course for over fifteen years, and we know what you need. This program can also be a great addition to your CDCES or BC-ADM exam study plan.
Download Course Schedule | Download Course Flyer
Join us LIVE for this Virtual Course and enjoy a sense of community!
Team of expert faculty includes:
- Diana Isaacs, PharmD, BCPS, BC-ADM, BCACP, CDCES – Educator of the Year, 2020
- Coach Beverly Thomassian, RN, MPH, CDCES, BC-ADM
- Ashley LaBrier, MS, RD, CDCES, Diabetes Program Coordinator
Two Registration Options
Virtual DiabetesEd Specialist Conference Deluxe | 30+ CEs
Deluxe Option for $499: Virtual Program includes:
- Q & A Session with the instructor after each webinar.
- LIVE Presentations by our team of experts.
- State of the art review of current diabetes care and technology.
- Resources for each session.
- Access to free podcasts and video recordings within a week of each live session for one year.
Deluxe Version includes Syllabus, Standards and Swag*:
- Diabetes Educator Course 2022 Syllabus Hard Copy – over 100 pages -This spiral-bound workbook contains the printed version of all of the instructor’s slides.
- ADA 2022 Standards of Care Book -The ADA Standards of Medical Care in Diabetes is a key resource for healthcare professionals involved in diabetes care, education, and support.
- DiabetesEd Services highlighters, Medication PocketCard, Tote Bag and Pen
Virtual DiabetesEd Specialist Conference Basic | 30+ CEs
Deluxe Option for $499: Virtual Program includes:
- Q & A Session with the instructor after each webinar.
- LIVE Presentations by our team of experts.
- State of the art review of current diabetes care and technology.
- Resources for each session.
- Access to free podcasts and video recordings within a week of each live session for one year.
Don’t worry if you can’t make it live. Your registration guarantees access to the recorded version in the Online University.
All hours earned count toward your CDCES Accreditation Information
Sign up for Diabetes Blog Bytes – we post one daily Blog Byte from Monday to Friday. And of course, Tuesday is our Question of the Week. It’s Informative and FREE! Sign up below!
[yikes-mailchimp form=”1″]The use of DES products does not guarantee the successful passage of the CDCES exam. CBDCE does not endorse any preparatory or review materials for the CDCES exam, except for those published by CBDCE.
















 Coach Beverly Thomassian RN, MPH, CDCES, BC-ADM – CEO of DiabetesEd Services
Coach Beverly Thomassian RN, MPH, CDCES, BC-ADM – CEO of DiabetesEd Services Diana Isaacs, PharmD, BCPS, BCACP, CDCES, BC-ADM, FADCES, FCCPCES
Diana Isaacs, PharmD, BCPS, BCACP, CDCES, BC-ADM, FADCES, FCCPCES  In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Diabetes Education Services. Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and Diabetes Education Services. Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.